Inner but not outer membrane leaflets control the transition from glycosylphosphatidylinositol-anchored influenza hemagglutinin-induced hemifusion to full fusion
- PMID: 9060465
- PMCID: PMC2132481
- DOI: 10.1083/jcb.136.5.995
Inner but not outer membrane leaflets control the transition from glycosylphosphatidylinositol-anchored influenza hemagglutinin-induced hemifusion to full fusion
Abstract
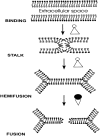
Cells that express wild-type influenza hemagglutinin (HA) fully fuse to RBCs, while cells that express the HA-ectodomain anchored to membranes by glycosylphosphatidylinositol, rather than by a transmembrane domain, only hemifuse to RBCs. Amphipaths were inserted into inner and outer membrane leaflets to determine the contribution of each leaflet in the transition from hemifusion to fusion. When inserted into outer leaflets, amphipaths did not promote the transition, independent of whether the agent induces monolayers to bend outward (conferring positive spontaneous monolayer curvature) or inward (negative curvature). In contrast, when incorporated into inner leaflets, positive curvature agents led to full fusion. This suggests that fusion is completed when a lipidic fusion pore with net positive curvature is formed by the inner leaflets that compose a hemifusion diaphragm. Suboptimal fusion conditions were established for RBCs bound to cells expressing wild-type HA so that lipid but not aqueous dye spread was observed. While this is the same pattern of dye spread as in stable hemifusion, for this "stunted" fusion, lower concentrations of amphipaths in inner leaflets were required to promote transfer of aqueous dyes. Also, these amphipaths induced larger pores for stunted fusion than they generated within a stable hemifusion diaphragm. Therefore, spontaneous curvature of inner leaflets can affect formation and enlargement of fusion pores induced by HA. We propose that after the HA-ectodomain induces hemifusion, the transmembrane domain causes pore formation by conferring positive spontaneous curvature to leaflets of the hemifusion diaphragm.
Figures

Similar articles
-
GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes.J Cell Biol. 1995 Nov;131(3):679-91. doi: 10.1083/jcb.131.3.679. J Cell Biol. 1995. PMID: 7593189 Free PMC article.
-
Meta-stability of the hemifusion intermediate induced by glycosylphosphatidylinositol-anchored influenza hemagglutinin.Biophys J. 1997 Nov;73(5):2280-91. doi: 10.1016/S0006-3495(97)78260-2. Biophys J. 1997. PMID: 9370425 Free PMC article.
-
Effects of spontaneous bilayer curvature on influenza virus-mediated fusion pores.J Gen Physiol. 1998 Oct;112(4):409-22. doi: 10.1085/jgp.112.4.409. J Gen Physiol. 1998. PMID: 9758860 Free PMC article.
-
Structural intermediates in influenza haemagglutinin-mediated fusion.Mol Membr Biol. 1999 Jan-Mar;16(1):33-42. doi: 10.1080/096876899294733. Mol Membr Biol. 1999. PMID: 10332735 Review.
-
Architecture of the influenza hemagglutinin membrane fusion site.Biochim Biophys Acta. 2003 Jul 11;1614(1):24-35. doi: 10.1016/s0005-2736(03)00160-3. Biochim Biophys Acta. 2003. PMID: 12873763 Review.
Cited by
-
Polybasic KKR motif in the cytoplasmic tail of Nipah virus fusion protein modulates membrane fusion by inside-out signaling.J Virol. 2007 May;81(9):4520-32. doi: 10.1128/JVI.02205-06. Epub 2007 Feb 14. J Virol. 2007. PMID: 17301148 Free PMC article.
-
The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement.J Membr Biol. 2004 May 1;199(1):1-14. doi: 10.1007/s00232-004-0669-8. J Membr Biol. 2004. PMID: 15366419 Review.
-
Role of Abl kinase and the Wave2 signaling complex in HIV-1 entry at a post-hemifusion step.PLoS Pathog. 2010 Jun 17;6(6):e1000956. doi: 10.1371/journal.ppat.1000956. PLoS Pathog. 2010. PMID: 20585556 Free PMC article.
-
The transmembrane domain and cytoplasmic tail of herpes simplex virus type 1 glycoprotein H play a role in membrane fusion.J Virol. 2002 Nov;76(21):10708-16. doi: 10.1128/jvi.76.21.10708-10716.2002. J Virol. 2002. PMID: 12368313 Free PMC article.
-
Kinetically differentiating influenza hemagglutinin fusion and hemifusion machines.Biophys J. 2003 Sep;85(3):1713-24. doi: 10.1016/S0006-3495(03)74601-3. Biophys J. 2003. PMID: 12944286 Free PMC article.
References
-
- Abidor IG, Arakelian VB, Chernomordik LV, Yu, Chizmadzhev A, Pastushenko VF, Tarasevich MR. Electrical breakdown of BLM: main experimental facts and their qualitative discussion. Bioelectrochem Bioenerg. 1979;6:37–52.
-
- Chernomordik LV, Melikyan GB, Yu, Chizmadzhev A. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta. 1987;906:309–352. - PubMed
-
- Chernomordik LV, Vogel SS, Leikina EA, Sokoloff A, Onoran HO, Zimmerberg J. Lysolipids reversibly inhibit Ca2+-, GTP- and pH- dependent fusion of biological membranes. FEBS Lett. 1993;318:71–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

