The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide-MHC molecule ligands
- PMID: 9016871
- PMCID: PMC2196122
- DOI: 10.1084/jem.185.2.219
The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide-MHC molecule ligands
Abstract
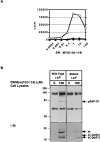
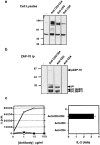
One hypothesis seeking to explain the signaling and biological properties of T cell receptor for antigen (TCR) partial agonists and antagonists is the coreceptor density/kinetic model, which proposes that the pharmacologic behavior of a TCR ligand is largely determined by the relative rates of (a) dissociation ofligand from an engaged TCR and (b) recruitment oflck-linked coreceptors to this ligand-engaged receptor. Using several approaches to prevent or reduce the association of CD4 with occupied TCR, we demonstrate that consistent with this hypothesis, the biological and biochemical consequence of limiting this interaction is to convert typical agonists into partial agonist stimuli. Thus, adding anti-CD4 antibody to T cells recognizing a wild-type peptide-MHC class II ligand leads to disproportionate inhibition of interleukin-2 (IL-2) relative to IL-3 production, the same pattern seen using a TCR partial agonist/antagonist. In addition, T cells exposed to wild-type ligand in the presence of anti-CD4 antibodies show a pattern of TCR signaling resembling that seen using partial agonists, with predominant accumulation of the p21 tyrosine-phosphorylated form of TCR-zeta, reduced tyrosine phosphorylation of CD3epsilon, and no detectable phosphorylation of ZAP-70. Similar results are obtained when the wild-type ligand is presented by mutant class II MHC molecules unable to bind CD4. Likewise, antibody coligation of CD3 and CD4 results in an agonist-like phosphorylation pattern, whereas bivalent engagement of CD3 alone gives a partial agonist-like pattern. Finally, in accord with data showing that partial agonists often induce T cell anergy, CD4 blockade during antigen exposure renders cloned T cells unable to produce IL-2 upon restimulation. These results demonstrate that the biochemical and functional responses to variant TCR ligands with partial agonist properties can be largely reproduced by inhibiting recruitment of CD4 to a TCR binding a wild-type ligand, consistent with the idea that the relative rates of TCR-ligand disengagement and of association of engaged TCR with CD4 may play a key role in determining the pharmacologic properties of peptide-MHC molecule ligands. Beyond this insight into signaling through the TCR, these results have implications for models of thymocyte selection and the use of anti-coreceptor antibodies in vivo for the establishment ofimmunological tolerance.
Figures




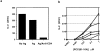
Similar articles
-
Dissociation of intracellular signaling pathways in response to partial agonist ligands of the T cell receptor.J Exp Med. 1998 May 18;187(10):1699-709. doi: 10.1084/jem.187.10.1699. J Exp Med. 1998. PMID: 9584148 Free PMC article.
-
Partial signaling by CD8+ T cells in response to antagonist ligands.J Exp Med. 1996 Jul 1;184(1):149-57. doi: 10.1084/jem.184.1.149. J Exp Med. 1996. PMID: 8691128 Free PMC article.
-
Serial TCR engagement and down-modulation by peptide:MHC molecule ligands: relationship to the quality of individual TCR signaling events.J Immunol. 1999 Feb 15;162(4):2073-80. J Immunol. 1999. PMID: 9973480
-
Variant TCR ligands: new insights into the molecular basis of antigen-dependent signal transduction and T-cell activation.Semin Immunol. 1996 Apr;8(2):83-101. doi: 10.1006/smim.1996.0011. Semin Immunol. 1996. PMID: 8920243 Review.
-
Suboptimal engagement of the T-cell receptor by a variety of peptide-MHC ligands triggers T-cell anergy.Immunology. 2010 Jan;129(1):1-7. doi: 10.1111/j.1365-2567.2009.03206.x. Epub 2009 Dec 2. Immunology. 2010. PMID: 20002785 Free PMC article. Review.
Cited by
-
Thymocyte apoptosis.J Clin Immunol. 1999 Nov;19(6):337-49. doi: 10.1023/a:1020594531159. J Clin Immunol. 1999. PMID: 10634208
-
Activation of macrophages and interference with CD4+ T-cell stimulation by Mycobacterium avium subspecies paratuberculosis and Mycobacterium avium subspecies avium.Immunology. 2003 Jan;108(1):62-9. doi: 10.1046/j.1365-2567.2003.01564.x. Immunology. 2003. PMID: 12519304 Free PMC article.
-
Reciprocal TCR-CD3 and CD4 Engagement of a Nucleating pMHCII Stabilizes a Functional Receptor Macrocomplex.Cell Rep. 2018 Jan 30;22(5):1263-1275. doi: 10.1016/j.celrep.2017.12.104. Cell Rep. 2018. PMID: 29386113 Free PMC article.
-
Dissociation of intracellular signaling pathways in response to partial agonist ligands of the T cell receptor.J Exp Med. 1998 May 18;187(10):1699-709. doi: 10.1084/jem.187.10.1699. J Exp Med. 1998. PMID: 9584148 Free PMC article.
-
Interplay between TCR affinity and necessity of coreceptor ligation: high-affinity peptide-MHC/TCR interaction overcomes lack of CD8 engagement.J Immunol. 2003 Nov 1;171(9):4493-503. doi: 10.4049/jimmunol.171.9.4493. J Immunol. 2003. PMID: 14568922 Free PMC article.
References
-
- Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-ζ and lack of ZAP-70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. - PubMed
-
- Madrenas J, Wange RL, Wang JL, Isakov NA, Samelson LE, Germain RN. ζ phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science (Wash DC) 1995;267:515–518. - PubMed
-
- Kersh GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature (Lond) 1996;380:495–498. - PubMed
-
- Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science (Wash DC) 1991;252:1308–1310. - PubMed
-
- De Magistris M, Alexander J, Coggeshall M, Altman A, Gaeta FC, Grey HM, Sette A. Antigen analog– major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68:625–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

