Selective reconstitution of gastrin-releasing peptide receptor with G alpha q
- PMID: 9012857
- PMCID: PMC19586
- DOI: 10.1073/pnas.94.2.751
Selective reconstitution of gastrin-releasing peptide receptor with G alpha q
Abstract
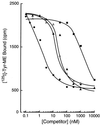
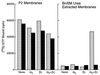
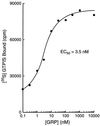
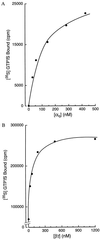
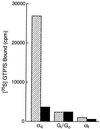
Identification of the molecular mechanisms that determine specificity of coupling interactions between gastrin-releasing peptide receptors (GRPrs) and their cognate heterotrimeric GTP-binding proteins is a fundamental step in understanding the signal transduction cascade initiated by receptor-ligand interaction. To explore these mechanisms in greater detail, we have developed an in situ reconstitution assay in chaotrope-extracted membranes from mouse fibroblasts expressing the GRPr, and we have used it to measure GRPr-catalyzed binding of GTP gamma S to purified G protein alpha subunits. Binding studies with 125I-labeled [D-Tyr6]bombesin(6-13) methyl ester (125I-Tyr-ME), a GRPr specific antagonist, show a single binding site with a Kd = 1.4 nM +/- 0.4 (mean +/- SD, n = 3) and capacity of 15-22 pmol of receptor per mg of protein in the extracted membrane preparations, representing a 2- to 3-fold enrichment of binding sites compared with the membranes before extraction. Quantitative ligand displacement analysis using various unlabeled GRPr agonists shows a rank order of potency characteristic of the GRPr: bombesin > or = GRP > > neuromedin B. Reconstitution of urea extracted membranes with a purified G alpha q showed that receptor-catalyzed binding of GTP gamma S was dependent on agonist (GRP) and G beta gamma subunits. The EC50 for GRP was 3.5 nM, which correlates well with the reported Kd of 3.1 nM for GRP binding to GRPr expressed in mouse fibroblasts [Benya, R. V., et al. (1994) Mol. Pharmacol. 46, 235-245]. The apparent Kd for bovine brain G beta gamma in this assay was 60 nM, and the Km for squid retinal G alpha q was 90 nM. The GRPr-catalyzed binding of GTP gamma S is selective for G alpha q, since we did not detect receptor-catalyzed exchange using either G alpha i/o or G alpha t. These data demonstrate that GRPr can functionally couple to G alpha q but not to the pertussis toxin-sensitive G alpha i/o or retinal specific G alpha t. This in situ receptor reconstitution method will allow molecular characterization of G protein coupling to other heptahelical receptors.
Figures
Similar articles
-
GRP-preferring bombesin receptors increase generation of inositol phosphates and tension in rat myometrium.Am J Physiol. 1993 Dec;265(6 Pt 1):C1579-87. doi: 10.1152/ajpcell.1993.265.6.C1579. Am J Physiol. 1993. PMID: 8279518
-
An aspartate residue at the extracellular boundary of TMII and an arginine residue in TMVII of the gastrin-releasing peptide receptor interact to facilitate heterotrimeric G protein coupling.Biochemistry. 1999 Jul 20;38(29):9366-72. doi: 10.1021/bi990544h. Biochemistry. 1999. PMID: 10413511
-
The bombesin receptor subtypes have distinct G protein specificities.J Biol Chem. 1999 Apr 23;274(17):11573-81. doi: 10.1074/jbc.274.17.11573. J Biol Chem. 1999. PMID: 10206964
-
Novel insight on GRP/GRPR axis in diseases.Biomed Pharmacother. 2023 May;161:114497. doi: 10.1016/j.biopha.2023.114497. Epub 2023 Mar 16. Biomed Pharmacother. 2023. PMID: 36933382 Review.
-
Role of the GRP/GRPR System in Regulating Brain Functions.ACS Chem Neurosci. 2023 Oct 4;14(19):3588-3598. doi: 10.1021/acschemneuro.3c00392. Epub 2023 Sep 13. ACS Chem Neurosci. 2023. PMID: 37702025 Review.
Cited by
-
Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils.Proc Natl Acad Sci U S A. 2012 Jan 10;109(2):547-52. doi: 10.1073/pnas.1110996109. Epub 2011 Dec 27. Proc Natl Acad Sci U S A. 2012. PMID: 22203955 Free PMC article.
-
Modulation of the interaction between neurotensin receptor NTS1 and Gq protein by lipid.J Mol Biol. 2012 Mar 16;417(1-2):95-111. doi: 10.1016/j.jmb.2012.01.023. Epub 2012 Jan 27. J Mol Biol. 2012. PMID: 22306739 Free PMC article.
-
Function of non-visual arrestins in signaling and endocytosis of the gastrin-releasing peptide receptor (GRP receptor).Biochem Pharmacol. 2008 Mar 1;75(5):1170-85. doi: 10.1016/j.bcp.2007.11.022. Epub 2007 Dec 8. Biochem Pharmacol. 2008. PMID: 18199425 Free PMC article.
-
Structural prerequisites for G-protein activation by the neurotensin receptor.Nat Commun. 2015 Jul 24;6:7895. doi: 10.1038/ncomms8895. Nat Commun. 2015. PMID: 26205105 Free PMC article.
-
Neuronal pentraxin 2 is required for facilitating excitatory synaptic inputs onto spinal neurons involved in pruriceptive transmission in a model of chronic itch.Nat Commun. 2022 May 2;13(1):2367. doi: 10.1038/s41467-022-30089-x. Nat Commun. 2022. PMID: 35501343 Free PMC article.
References
-
- Lebacq-Verheyden A-M, Trepel J, Sausville E A, Battey J F. In: Handbook of Experimental Pharmacology. Sporn M, Roberts A, editors. Vol. 95. Berlin: Springer; 1990. pp. 71–124.
-
- Spindel E R. Trends Neurosci. 1986;9:130–133.
-
- Bold R J, Lowry P S, Ishizuka J, Battey J F, Townsend C M, Jr, Thompson J C. J Cell Physiol. 1994;161:519–525. - PubMed
-
- Bologna M, Festuccia C, Muzi P, Biordi L, Ciomei M. Cancer. 1989;63:1714–1720. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

