Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice
- PMID: 8976182
- PMCID: PMC2196394
- DOI: 10.1084/jem.184.6.2271
Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice
Abstract
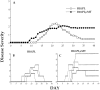
Experimental autoimmune encephalomyelitis (EAE) is an animal model for autoimmune central nervous system disease mediated by CD4 T cells. To examine the role of B cells in the induction of EAE, we used B10.PL (I-Au) mice rendered deficient in B cells by deletion of their mu chain transmembrane region (B10.PLmicroMT). By immunizing B10.PL and B10.PLmicroMT mice with the NH-terminal myelin basic protein encephalitogenic peptide Ac1-11, we observed no difference in the onset or severity of disease in the absence of mature B cells. There was, however, a greater variation in disease onset, severity, and especially of recovery in the B cell-deficient mice compared to controls. B10.PLmicroMT mice rarely returned to normal in the absence of B cells. Taken together, our data suggest that B cells do not play a role in the activation of encephalitogenic T cells, but may contribute to the immune modulation of acute EAE. The mechanisms to explain these effects are discussed.
Figures

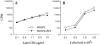
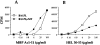
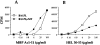
Similar articles
-
Relapsing and remitting experimental autoimmune encephalomyelitis in B cell deficient mice.J Autoimmun. 2000 Jun;14(4):311-8. doi: 10.1006/jaut.2000.0371. J Autoimmun. 2000. PMID: 10882057
-
Induction of a heterogeneous TCR repertoire in (PL/JXSJL/J)F1 mice by myelin basic protein peptide Ac1-11 and its analog Ac1-11[4A].Mol Immunol. 1997 Aug;34(11):781-92. doi: 10.1016/s0161-5890(97)00058-8. Mol Immunol. 1997. PMID: 9444977
-
Expression of the tyrosine phosphatase SRC homology 2 domain-containing protein tyrosine phosphatase 1 determines T cell activation threshold and severity of experimental autoimmune encephalomyelitis.J Immunol. 2002 May 1;168(9):4511-8. doi: 10.4049/jimmunol.168.9.4511. J Immunol. 2002. PMID: 11970996
-
Inactivation of T cell receptor peptide-specific CD4 regulatory T cells induces chronic experimental autoimmune encephalomyelitis (EAE).J Exp Med. 1996 Nov 1;184(5):1609-17. doi: 10.1084/jem.184.5.1609. J Exp Med. 1996. PMID: 8920851 Free PMC article.
-
Preferential distribution of V beta 8.2-positive T cells in the central nervous system of rats with myelin basic protein-induced autoimmune encephalomyelitis.Eur J Immunol. 1993 Oct;23(10):2399-406. doi: 10.1002/eji.1830231004. Eur J Immunol. 1993. PMID: 7691605
Cited by
-
Role of B cells in tolerance induction.Curr Opin Organ Transplant. 2015 Aug;20(4):369-75. doi: 10.1097/MOT.0000000000000204. Curr Opin Organ Transplant. 2015. PMID: 26107970 Free PMC article. Review.
-
Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis.Arthritis Res Ther. 2012 Feb 8;14(1):R32. doi: 10.1186/ar3736. Arthritis Res Ther. 2012. PMID: 22315945 Free PMC article.
-
IL-10-independent regulatory B-cell subsets and mechanisms of action.Int Immunol. 2015 Oct;27(10):531-6. doi: 10.1093/intimm/dxv033. Epub 2015 May 20. Int Immunol. 2015. PMID: 25999596 Free PMC article. Review.
-
Role of regulatory b cells in neuroimmunologic disorders.J Neurosci Res. 2016 Aug;94(8):693-701. doi: 10.1002/jnr.23749. Epub 2016 Apr 26. J Neurosci Res. 2016. PMID: 27112131 Free PMC article. Review.
-
Is there a feudal hierarchy amongst regulatory immune cells? More than just Tregs.Arthritis Res Ther. 2009;11(4):237. doi: 10.1186/ar2752. Epub 2009 Aug 4. Arthritis Res Ther. 2009. PMID: 19664198 Free PMC article. Review.
References
-
- Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995;32:121–182. - PubMed
-
- Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–199. - PubMed
-
- Zamil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. - PubMed
-
- Fritz RB, Skeen MJ, Jen-Chou CH, Garcia M, Egorov IK. Major histocompatibility complex–linked control of the murine immune response to myelin basic protein. J Immunol. 1985;134:2328–2332. - PubMed
-
- Martin R, McFarland HF, McFarlin DE. Immunology of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

