Dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity in apolipoprotein A-II knockout mice suggest a complex role for apolipoprotein A-II in atherosclerosis susceptibility
- PMID: 8962133
- PMCID: PMC26214
- DOI: 10.1073/pnas.93.25.14788
Dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity in apolipoprotein A-II knockout mice suggest a complex role for apolipoprotein A-II in atherosclerosis susceptibility
Abstract
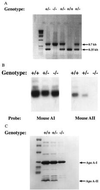
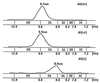
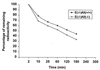
Apolipoprotein (apo) A-II is the second most abundant apolipoprotein in high density lipoprotein (HDL). To study its role in lipoprotein metabolism and atherosclerosis susceptibility, apo A-II knockout mice were created. Homozygous knockout mice had 67% and 52% reductions in HDL cholesterol levels in the fasted and fed states, respectively, and HDL particle size was reduced. Metabolic turnover studies revealed the HDL decrease to be due to both decreased HDL cholesterol ester and apo A-I transport rate and increased HDL cholesterol ester and apo A-I fractional catabolic rate. The apo A-II deficiency trait was bred onto the atherosclerosis-prone apo E-deficient background, which resulted in a surprising 66% decrease in cholesterol levels due primarily to decreased atherogenic lipoprotein remnant particles. Metabolic turnover studies indicated increased remnant clearance in the absence of apo A-II. Finally, apo A-II deficiency was associated with lower free fatty acid, glucose, and insulin levels, suggesting an insulin hypersensitivity state. In summary, apo A-II plays a complex role in lipoprotein metabolism, with some antiatherogenic properties such as the maintenance of a stable HDL pool, and other proatherogenic properties such as decreasing clearance of atherogenic lipoprotein remnants and promotion of insulin resistance.
Figures



Similar articles
-
Apolipoprotein A-II, HDL metabolism and atherosclerosis.Atherosclerosis. 2002 Sep;164(1):1-13. doi: 10.1016/s0021-9150(01)00751-1. Atherosclerosis. 2002. PMID: 12119188 Review.
-
Substitution of the carboxyl-terminal domain of apo AI with apo AII sequences restores the potential of HDL to reduce the progression of atherosclerosis in apo E knockout mice.J Clin Invest. 1998 Jul 15;102(2):379-85. doi: 10.1172/JCI3038. J Clin Invest. 1998. PMID: 9664079 Free PMC article.
-
Decreased susceptibility to diet-induced atherosclerosis in human apolipoprotein A-II transgenic mice.Arterioscler Thromb Vasc Biol. 2000 Nov;20(11):2453-8. doi: 10.1161/01.atv.20.11.2453. Arterioscler Thromb Vasc Biol. 2000. PMID: 11073852
-
Pharmacological modulation of cholesteryl ester transfer protein, a new therapeutic target in atherogenic dyslipidemia.Pharmacol Ther. 2004 Jan;101(1):17-38. doi: 10.1016/j.pharmthera.2003.10.001. Pharmacol Ther. 2004. PMID: 14729390 Review.
-
Familial apolipoprotein AI and apolipoprotein CIII deficiency. Subclass distribution, composition, and morphology of lipoproteins in a disorder associated with premature atherosclerosis.J Clin Invest. 1984 Nov;74(5):1601-13. doi: 10.1172/JCI111576. J Clin Invest. 1984. PMID: 6501564 Free PMC article.
Cited by
-
Impact of genetic deletion of platform apolipoproteins on the size distribution of the murine lipoproteome.J Proteomics. 2016 Sep 2;146:184-94. doi: 10.1016/j.jprot.2016.06.035. Epub 2016 Jul 3. J Proteomics. 2016. PMID: 27385375 Free PMC article.
-
Quantitative trait locus analysis of atherosclerosis in an intercross between C57BL/6 and C3H mice carrying the mutant apolipoprotein E gene.Genetics. 2006 Mar;172(3):1799-807. doi: 10.1534/genetics.105.051912. Epub 2005 Dec 30. Genetics. 2006. PMID: 16387874 Free PMC article.
-
A phenotype-sensitizing Apoe-deficient genetic background reveals novel atherosclerosis predisposition loci in the mouse.Genetics. 2002 Apr;160(4):1599-608. doi: 10.1093/genetics/160.4.1599. Genetics. 2002. PMID: 11973313 Free PMC article.
-
Apolipoprotein A-II, a Player in Multiple Processes and Diseases.Biomedicines. 2022 Jul 2;10(7):1578. doi: 10.3390/biomedicines10071578. Biomedicines. 2022. PMID: 35884883 Free PMC article. Review.
-
Niacin, an old drug with a new twist.J Lipid Res. 2013 Oct;54(10):2586-94. doi: 10.1194/jlr.R040592. Epub 2013 Aug 15. J Lipid Res. 2013. PMID: 23948546 Free PMC article. Review.
References
-
- Miller N E. Am Heart J. 1987;113:589–597. - PubMed
-
- Castelli W P, Garrison R J, Wilson P W F, Abbott R D, Kalousdian S, Kannel W B. J Am Med Assoc. 1986;256:2835–2838. - PubMed
-
- Gordon T, Castelli W P, Hjortland M C, Kannel W B, Dawber T R. Am J Med. 1977;62:701–714. - PubMed
-
- Gordon D J, Probstfield J L, Garrison R J, Neaton J D, Castelli W P, Knoke J D, Jacobs D R, Jr, Bangdiwala S, Tyroler H A. Circulation. 1989;79:8–15. - PubMed
-
- Eisenberg S. J Lipid Res. 1984;25:1017–1058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

