Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene
- PMID: 8756432
- PMCID: PMC6579310
- DOI: 10.1523/JNEUROSCI.16-16-05014.1996
Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene
Abstract
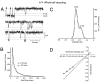
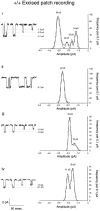
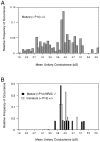
A novel strain of mutant mouse has been generated with a deletion of the gene encoding the NR2C subunit of the NMDA receptor, which is primarily expressed in cerebellar granule cells. Patch-clamp recordings from granule cells in thin cerebellar slices were used to assess the consequences of the gene deletion. In granule cells of wild-type animals, a wide range of single-channel conductances were observed (19-60 pS). The disruption of the NR2C gene results in the disappearance of low-conductance NMDA receptor channels ( < 37 pS) normally expressed in granule cells during developmental maturation. The NMDA receptor-mediated synaptic current is markedly potentiated in amplitude, but abbreviated in duration (with no net difference in total charge), and the non-NMDA component of the synaptic current was reduced. We conclude that the NR2C subunit contributes to functional heteromeric NMDA receptor-subunit assemblies at the mossy fiber synapse and extrasynaptic sites during maturation, and the conductance level exhibited by a given receptor macromolecule may reflect the stochiometry of subunit composition.
Figures






Similar articles
-
A direct comparison of the single-channel properties of synaptic and extrasynaptic NMDA receptors.J Neurosci. 1997 Jan 1;17(1):107-16. doi: 10.1523/JNEUROSCI.17-01-00107.1997. J Neurosci. 1997. PMID: 8987740 Free PMC article.
-
NMDA receptor 2 (NR2) C-terminal control of NR open probability regulates synaptic transmission and plasticity at a cerebellar synapse.J Neurosci. 2002 Nov 15;22(22):9687-97. doi: 10.1523/JNEUROSCI.22-22-09687.2002. J Neurosci. 2002. PMID: 12427824 Free PMC article.
-
Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit.J Neurosci. 1996 Dec 15;16(24):7859-67. doi: 10.1523/JNEUROSCI.16-24-07859.1996. J Neurosci. 1996. PMID: 8987814 Free PMC article.
-
NMDA receptor-mediated currents in rat cerebellar granule and unipolar brush cells.J Neurophysiol. 2002 Apr;87(4):1948-59. doi: 10.1152/jn.00599.2001. J Neurophysiol. 2002. PMID: 11929914
-
[Knockout mouse--its characteristics and application (1): NMDA receptor subunit knockout mouse].Nihon Shinkei Seishin Yakurigaku Zasshi. 1997 Oct;17(5):185-92. Nihon Shinkei Seishin Yakurigaku Zasshi. 1997. PMID: 9483578 Review. Japanese.
Cited by
-
A direct comparison of the single-channel properties of synaptic and extrasynaptic NMDA receptors.J Neurosci. 1997 Jan 1;17(1):107-16. doi: 10.1523/JNEUROSCI.17-01-00107.1997. J Neurosci. 1997. PMID: 8987740 Free PMC article.
-
NMDA receptor 2 (NR2) C-terminal control of NR open probability regulates synaptic transmission and plasticity at a cerebellar synapse.J Neurosci. 2002 Nov 15;22(22):9687-97. doi: 10.1523/JNEUROSCI.22-22-09687.2002. J Neurosci. 2002. PMID: 12427824 Free PMC article.
-
Essential role of NMDA receptor channel ε4 subunit (GluN2D) in the effects of phencyclidine, but not methamphetamine.PLoS One. 2010 Oct 28;5(10):e13722. doi: 10.1371/journal.pone.0013722. PLoS One. 2010. PMID: 21060893 Free PMC article.
-
Brainstem Modulates Parkinsonism-Induced Orofacial Sensorimotor Dysfunctions.Int J Mol Sci. 2023 Jul 31;24(15):12270. doi: 10.3390/ijms241512270. Int J Mol Sci. 2023. PMID: 37569642 Free PMC article.
-
Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer.J Neurosci. 1999 Jul 15;19(14):6027-36. doi: 10.1523/JNEUROSCI.19-14-06027.1999. J Neurosci. 1999. PMID: 10407040 Free PMC article.
References
-
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. - PubMed
-
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Bradley A. Production and analysis of chimeric mice. In: Robertson EJ, editor. Teratocarcinomas and embryonic stem cells: a practical approach. IRL; Oxford: 1987. pp. 113–151.
-
- Chazot PL, Coleman SK, Cik M, Stephenson FA. Molecular characterization of N -methyl-d-aspartate receptors expressed in mammalian cells yields evidence for the coexistence of three subunit types within a discrete receptor molecule. J Biol Chem. 1994;269:24403–24409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
