IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7-1 expression
- PMID: 8617961
- PMCID: PMC1952187
IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7-1 expression
Abstract
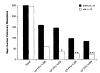
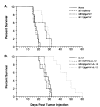
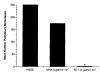
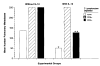
A number of cytokines and costimulatory molecules involved in immune activation have recently been identified including IL-12, a heterodimeric cytokine that supports the development of cell-mediated immunity, and B7-1, a costimulatory molecule involved in the activation of T lymphocytes. We explored the use of these immunomodulants as molecularly defined adjuvants in the function of recombinant anticancer vaccines using a murine model adenocarcinoma, CT26, transduced with a model Ag, beta-galactosidase (beta-gal). Although IL-12 given alone to mice bearing tumors established for 3 days did not have consistent antitumor activity, a profound therapeutic effect was observed when IL-12 administration was combined with a recombinant vaccinia virus (rVV) encoding beta-gal called VJS6. On the basis of the reported synergistic effects of IL-12 and the costimulatory molecule B7-1 (CD80) in vitro, we immunized mice with a double recombinant vaccinia encoding both the model tumor Ag and the costimulatory molecule B7-1, designated B7-1 beta-gal rVV. The adjuvant administration of IL-12 after immunization with this virus significantly enhanced survival in tumor-bearing animals. T cell subset depletions demonstrated that the in vivo activity of IL-12 was largely independent of CD4+ T lymphocytes, whereas the in vivo activity of a B7-1 rVV required both CD4+ and CD8+ T cells to elicit maximal therapeutic effect. To our knowledge, this is the first description of B7-1 and IL-12 cooperation in vivo and represents a novel strategy to enhance the efficacy of recombinant anticancer vaccines.
Figures


Similar articles
-
Construction and characterization of a triple-recombinant vaccinia virus encoding B7-1, interleukin 12, and a model tumor antigen.J Natl Cancer Inst. 1998 Dec 16;90(24):1881-7. doi: 10.1093/jnci/90.24.1881. J Natl Cancer Inst. 1998. PMID: 9862625 Free PMC article.
-
Costimulation enhances the active immunotherapy effect of recombinant anticancer vaccines.Cancer Res. 1996 Jun 15;56(12):2832-6. Cancer Res. 1996. PMID: 8665522 Free PMC article.
-
IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases.J Immunol. 1995 May 15;154(10):5282-92. J Immunol. 1995. PMID: 7730632 Free PMC article.
-
The next wave of recombinant and synthetic anticancer vaccines.Semin Cancer Biol. 1995 Dec;6(6):337-47. doi: 10.1016/1044-579x(95)90003-9. Semin Cancer Biol. 1995. PMID: 8938272 Free PMC article. Review.
-
IL-12: a key cytokine in immune regulation.Immunol Today. 1996 May;17(5):214-7. doi: 10.1016/0167-5699(96)30011-x. Immunol Today. 1996. PMID: 8991382 Review.
Cited by
-
Role of interleukin-12 in the regulation of CD4+ T cell apoptosis in a mouse model of asthma.Clin Exp Immunol. 2003 Feb;131(2):199-205. doi: 10.1046/j.1365-2249.2003.02073.x. Clin Exp Immunol. 2003. PMID: 12562378 Free PMC article.
-
The new vaccines: building viruses that elicit antitumor immunity.Curr Opin Immunol. 1996 Oct;8(5):658-63. doi: 10.1016/s0952-7915(96)80082-3. Curr Opin Immunol. 1996. PMID: 8902391 Free PMC article. Review.
-
The employment of vaccinia virus for colorectal cancer treatment: A review of preclinical and clinical studies.Hum Vaccin Immunother. 2022 Nov 30;18(6):2143698. doi: 10.1080/21645515.2022.2143698. Epub 2022 Nov 11. Hum Vaccin Immunother. 2022. PMID: 36369829 Free PMC article. Review.
-
Construction and characterization of a triple-recombinant vaccinia virus encoding B7-1, interleukin 12, and a model tumor antigen.J Natl Cancer Inst. 1998 Dec 16;90(24):1881-7. doi: 10.1093/jnci/90.24.1881. J Natl Cancer Inst. 1998. PMID: 9862625 Free PMC article.
-
Oncolytic virus therapy for cancer.Oncolytic Virother. 2013 Sep 23;2:31-46. doi: 10.2147/OV.S38901. eCollection 2013. Oncolytic Virother. 2013. PMID: 27512656 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
