Identification of potent, selective P2Y-purinoceptor agonists: structure-activity relationships for 2-thioether derivatives of adenosine 5'-triphosphate
- PMID: 8254622
- PMCID: PMC4431635
- DOI: 10.1021/jm00076a023
Identification of potent, selective P2Y-purinoceptor agonists: structure-activity relationships for 2-thioether derivatives of adenosine 5'-triphosphate
Abstract
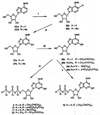
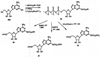
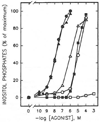
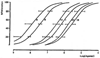
Study of P2-purinoceptor subtypes has been difficult due to the lack of potent and selective ligands. With the goal of developing high affinity P2-purinoceptor-selective agonist, we have synthesized a series of analogues of adenine nucleotides modified on the purine ring as chain-extended 2-thioethers or as N6-methyl-substituted compounds. Chemical functionality incorporated in the thioether moiety included cyanoalkyl, nitroaromatic, amino, thiol, cycloalkyl, n-alkyl, and olefinic groups. Apparent affinity of the compounds for P2Y-purinoceptors was established by measurement of P2Y-purinoceptor-promoted phospholipase C activity in turkey erythrocyte membranes and relaxation of carbachol-contracted smooth muscle in three different preparations (guinea pig taenia coli, rabbit aorta, and rabbit mesenteric artery). Activity at P2X-purinoceptors was established by measurement of contraction of rabbit saphenous artery and of the guinea pig vas deferens and urinary bladder. All 11 of the 2-thioethers of ATP stimulated the production of inositol phosphates with K0.5 values of 1.5-770 nM, with an (aminophenyl)ethyl derivative being most potent. Two adenosine diphosphate analogues were equipotent to the corresponding ATP analogues. Adenosine monophosphate analogues were full agonists, although generally 4 orders of magnitude less potent. ATP 2-thioethers displayed pD2 values in the range of 6-8 in smooth muscle assay systems for activity at P2Y-receptors. There was a significant correlation for the 2-thioether compounds between the pK0.5 values for inositol phosphate production and the pD2 values for relaxation mediated via the P2Y-purinoceptors in the guinea pig taenia coli, but not for the vascular P2Y-receptors or for the P2X-receptors. At P2X-receptors, no activity was observed in the rabbit saphenous artery, but variable degrees of activity were observed in the guinea pig vas deferens and bladder depending on distal substituents of the thioether moiety. N6-Methyl-ATP was inactive at P2X-receptors, and approximately equipotent to ATP at taenia coil P2Y-receptors. This suggested that hybrid N6-methyl and 2-thioether ATP derivatives might be potent and selective for certain P2Y-receptors, as was shown for one such derivative, N6-methyl-2-(5-hexenylthio)-ATP.
Figures



Similar articles
-
Inhibitory action of PPADS on relaxant responses to adenine nucleotides or electrical field stimulation in guinea-pig taenia coli and rat duodenum.Br J Pharmacol. 1995 Aug;115(8):1509-17. doi: 10.1111/j.1476-5381.1995.tb16644.x. Br J Pharmacol. 1995. PMID: 8564212 Free PMC article.
-
Differential effects of suramin on P2-purinoceptors mediating contraction of the guinea-pig vas deferens and urinary bladder.Br J Pharmacol. 1994 May;112(1):219-25. doi: 10.1111/j.1476-5381.1994.tb13055.x. Br J Pharmacol. 1994. PMID: 8032645 Free PMC article.
-
Activation of P1- and P2Y-purinoceptors by ADP-ribose in the guinea-pig taenia coli, but not of P2X-purinoceptors in the vas deferens.Br J Pharmacol. 1992 Oct;107(2):367-74. doi: 10.1111/j.1476-5381.1992.tb12753.x. Br J Pharmacol. 1992. PMID: 1422586 Free PMC article.
-
Design and pharmacology of selective P2-purinoceptor antagonists.J Auton Pharmacol. 1996 Dec;16(6):341-4. doi: 10.1111/j.1474-8673.1996.tb00049.x. J Auton Pharmacol. 1996. PMID: 9131412 Review.
-
Purinoceptors: are there families of P2X and P2Y purinoceptors?Pharmacol Ther. 1994;64(3):445-75. doi: 10.1016/0163-7258(94)00048-4. Pharmacol Ther. 1994. PMID: 7724657 Review.
Cited by
-
Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y(1) receptor ligands.J Med Chem. 2000 Mar 9;43(5):829-42. doi: 10.1021/jm990249v. J Med Chem. 2000. PMID: 10715151 Free PMC article.
-
Structure-activity relationships of bisphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists.J Med Chem. 1999 May 6;42(9):1625-38. doi: 10.1021/jm980657j. J Med Chem. 1999. PMID: 10229631 Free PMC article.
-
Pivotal role of phosphate chain length in vasoconstrictor versus vasodilator actions of adenine dinucleotides in rat mesenteric arteries.J Physiol. 1995 Mar 15;483 ( Pt 3)(Pt 3):703-13. doi: 10.1113/jphysiol.1995.sp020615. J Physiol. 1995. PMID: 7776252 Free PMC article.
-
A Photo-clickable ATP-Mimetic Reveals Nucleotide Interactors in the Membrane Proteome.Cell Chem Biol. 2020 Aug 20;27(8):1073-1083.e12. doi: 10.1016/j.chembiol.2020.05.010. Epub 2020 Jun 9. Cell Chem Biol. 2020. PMID: 32521230 Free PMC article.
-
Fluorescent N2,N3-epsilon-adenine nucleoside and nucleotide probes: synthesis, spectroscopic properties, and biochemical evaluation.Chembiochem. 2006 Sep;7(9):1361-74. doi: 10.1002/cbic.200600070. Chembiochem. 2006. PMID: 16871613 Free PMC article.
References
-
- Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen. Pharmacol. 1985;16:433–440. - PubMed
-
- Hoyle CHV, Burnstock G. ATP receptors and their physiological roles. In: Stone TW, editor. Adenosine in the Nervous System. London: Academic Press Ltd; 1991. pp. 43–76.
-
- O'Connor SE, Dainty IA, Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol. Sci. 1991;12:137–141. - PubMed
-
- Evans RJ, Derkach V, Suprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;57:503–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
