Search for new purine- and ribose-modified adenosine analogues as selective agonists and antagonists at adenosine receptors
- PMID: 7707320
- PMCID: PMC3457658
- DOI: 10.1021/jm00007a014
Search for new purine- and ribose-modified adenosine analogues as selective agonists and antagonists at adenosine receptors
Abstract
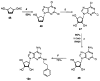
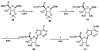
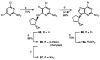
The binding affinities at rat A1, A2a, and A3 adenosine receptors of a wide range of derivatives of adenosine have been determined. Sites of modification include the purine moiety (1-, 3-, and 7-deaza; halo, alkyne, and amino substitutions at the 2- and 8-positions; and N6-CH2-ring, -hydrazino, and -hydroxylamino) and the ribose moiety (2'-, 3'-, and 5'-deoxy; 2'- and 3'- O-methyl; 2'-deoxy 2'-fluoro; 6'-thio; 5'-uronamide; carbocyclic; 4'- or 3'-methyl; and inversion of configuration). (-)- and (+)-5'-Noraristeromycin were 48- and 21-fold selective, respectively, for A2a vs A1 receptors. 2-Chloro-6'-thioadenosine displayed a Ki value of 20 nM at A2a receptors (15-fold selective vs A1). 2-Chloroadenin-9-yl(beta-L-2'-deoxy-6'- thiolyxofuranoside) displayed a Ki value of 8 microM at A1 receptors and appeared to be an antagonist, on the basis of the absence of a GTP-induced shift in binding vs a radiolabeled antagonist (8-cyclopentyl-1,3-dipropyl-xanthine). 2-Chloro-2'-deoxyadenosine and 2-chloroadenin-9-yl(beta-D-6'-thioarabinoside) were putative partial agonists at A1 receptors, with Ki values of 7.4 and 5.4 microM, respectively. The A2a selective agonist 2-(1-hexynyl)-5'-(N-ethylcarbamoyl)adenosine displayed a Ki value of 26 nM at A3 receptors. The 4'-methyl substitution of adenosine was poorly tolerated, yet when combined with other favorable modifications, potency was restored. Thus, N6-benzyl-4'-methyladenosine-5'-(N-methyluronamide) displayed a Ki value of 604 nM at A3 receptors and was 103- and 88-fold selective vs A1 and A2a receptors, respectively. This compound was a full agonist in the A3-mediated inhibition of adenylate cyclase in transfected CHO cells. The carbocyclic analogue of N6-(3-iodobenzyl)adenosine-5'-(N-methyluronamide) was 2-fold selective for A3 vs A1 receptors and was nearly inactive at A2a receptors.
Figures


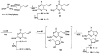
Similar articles
-
Structure-activity relationships of 9-alkyladenine and ribose-modified adenosine derivatives at rat A3 adenosine receptors.J Med Chem. 1995 May 12;38(10):1720-35. doi: 10.1021/jm00010a017. J Med Chem. 1995. PMID: 7752196 Free PMC article.
-
2-Substitution of N6-benzyladenosine-5'-uronamides enhances selectivity for A3 adenosine receptors.J Med Chem. 1994 Oct 14;37(21):3614-21. doi: 10.1021/jm00047a018. J Med Chem. 1994. PMID: 7932588 Free PMC article.
-
Interaction of 1,4-dihydropyridine and pyridine derivatives with adenosine receptors: selectivity for A3 receptors.J Med Chem. 1996 Jul 19;39(15):2980-9. doi: 10.1021/jm9600205. J Med Chem. 1996. PMID: 8709132 Free PMC article.
-
Adenosine A3 receptors: novel ligands and paradoxical effects.Trends Pharmacol Sci. 1998 May;19(5):184-91. doi: 10.1016/s0165-6147(98)01203-6. Trends Pharmacol Sci. 1998. PMID: 9652191 Free PMC article. Review.
-
Interactions of flavones and other phytochemicals with adenosine receptors.Adv Exp Med Biol. 2002;505:163-71. doi: 10.1007/978-1-4757-5235-9_15. Adv Exp Med Biol. 2002. PMID: 12083460 Free PMC article. Review.
Cited by
-
EFA (9-beta-D-erythrofuranosyladenine) is an effective salvage agent for methylthioadenosine phosphorylase-selective therapy of T-cell acute lymphoblastic leukemia with L-alanosine.Blood. 2006 Feb 1;107(3):898-903. doi: 10.1182/blood-2005-06-2430. Epub 2005 Oct 18. Blood. 2006. PMID: 16234352 Free PMC article.
-
Structure-based approaches to ligands for G-protein-coupled adenosine and P2Y receptors, from small molecules to nanoconjugates.J Med Chem. 2013 May 23;56(10):3749-67. doi: 10.1021/jm400422s. Epub 2013 May 9. J Med Chem. 2013. PMID: 23597047 Free PMC article. Review.
-
Nucleosides and emerging viruses: A new story.Drug Discov Today. 2022 Jul;27(7):1945-1953. doi: 10.1016/j.drudis.2022.02.013. Epub 2022 Feb 19. Drug Discov Today. 2022. PMID: 35189369 Free PMC article. Review.
-
Induction of apoptosis in HL-60 human promyelocytic leukemia cells by adenosine A(3) receptor agonists.Biochem Biophys Res Commun. 1996 Feb 27;219(3):904-10. doi: 10.1006/bbrc.1996.0331. Biochem Biophys Res Commun. 1996. PMID: 8645277 Free PMC article.
-
Medicinal chemistry of the A3 adenosine receptor: agonists, antagonists, and receptor engineering.Handb Exp Pharmacol. 2009;(193):123-59. doi: 10.1007/978-3-540-89615-9_5. Handb Exp Pharmacol. 2009. PMID: 19639281 Free PMC article. Review.
References
-
- Olsson RA, Pearson JD. Cardiovascular purinoceptors. Pharmacol Rev. 1990;3:761–845. - PubMed
-
- von Lubitz DKJE, Jacobson KA. Behavioral effects of adenosine receptor stimulation. In: Bellardinelli L, Pelleg A, editors. Adenosine and Adenine Nucleotides: From Molecular Biology to Integrative Physiology. Kluwer; Norwell, MA: 1995. pp. 489–498.
-
- Linden J. Cloned adenosine A3 receptors: Pharmacological properties, species differences and receptor function. Trends Pharmacol Sci. 1994;15:298–306. - PubMed
-
- Stiles GL. Adenosine receptors. J Biol Chem. 1992;267:6451–6454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
