The integrin receptor alpha 8 beta 1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C
- PMID: 7541634
- PMCID: PMC2692383
- DOI: 10.1016/0896-6273(95)90268-6
The integrin receptor alpha 8 beta 1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C
Abstract
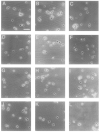
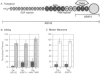
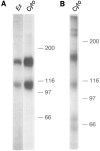
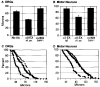
This paper identifies a neuronal receptor for tenascin-C (tenascin/cytotactin), an extracellular matrix protein that has previously been detected in developing sensory and motor neuron pathways and has been shown to regulate cell migration in the developing CNS. Antibodies specific for each subunit of the integrin alpha 8 beta 1 are used to demonstrate that alpha 8 beta 1 mediates neurite outgrowth of embryonic sensory and motor neurons on this extracellular matrix protein. In addition, expression of alpha 8 in K562 cells results in surface expression of alpha 8 beta 1 heterodimers that are shown to promote attachment of this cell line to tenascin. The major domain in tenascin that mediates neurite outgrowth is shown to be localized to fibronectin type III repeats 6-8.
Figures



Similar articles
-
Beta 8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV, and fibronectin.Mol Biol Cell. 1995 Apr;6(4):419-31. doi: 10.1091/mbc.6.4.419. Mol Biol Cell. 1995. PMID: 7542940 Free PMC article.
-
Dual function of tenascin: simultaneous promotion of neurite growth and inhibition of glial migration.J Cell Sci. 1993 Oct;106 ( Pt 2):597-610. doi: 10.1242/jcs.106.2.597. J Cell Sci. 1993. PMID: 7506709
-
Separate cell binding sites within cytotactin/tenascin differentially promote neurite outgrowth.Cell Adhes Commun. 1995 Aug;3(3):257-71. doi: 10.3109/15419069509081291. Cell Adhes Commun. 1995. PMID: 8846026
-
Tenascin-C in peripheral nerve morphogenesis.Perspect Dev Neurobiol. 1994;2(1):67-74. Perspect Dev Neurobiol. 1994. PMID: 7530145 Review.
-
Tenascin-contactin/F11 interactions: a clue for a developmental role?Perspect Dev Neurobiol. 1994;2(1):43-52. Perspect Dev Neurobiol. 1994. PMID: 7530143 Review.
Cited by
-
Proregenerative properties of ECM molecules.Biomed Res Int. 2013;2013:981695. doi: 10.1155/2013/981695. Epub 2013 Sep 9. Biomed Res Int. 2013. PMID: 24195084 Free PMC article. Review.
-
Cell-adhesive responses to tenascin-C splice variants involve formation of fascin microspikes.Mol Biol Cell. 1997 Oct;8(10):2055-75. doi: 10.1091/mbc.8.10.2055. Mol Biol Cell. 1997. PMID: 9348542 Free PMC article.
-
Integrins as receptor targets for neurological disorders.Pharmacol Ther. 2012 Apr;134(1):68-81. doi: 10.1016/j.pharmthera.2011.12.008. Epub 2011 Dec 30. Pharmacol Ther. 2012. PMID: 22233753 Free PMC article. Review.
-
Regionally specified human pluripotent stem cell-derived astrocytes exhibit different molecular signatures and functional properties.Development. 2019 Jul 8;146(13):dev170910. doi: 10.1242/dev.170910. Development. 2019. PMID: 31189664 Free PMC article.
-
Comparison of neurite outgrowth induced by intact and injured sciatic nerves: a confocal and functional analysis.J Neurosci. 1998 Jan 1;18(1):328-38. doi: 10.1523/JNEUROSCI.18-01-00328.1998. J Neurosci. 1998. PMID: 9412511 Free PMC article.
References
-
- Burns FR, von Kannen S, Guy L, Raper JA, Kamholz J, Chang S. DM-GRASP, a novel immunoglobulin superfamily axonal surface protein that supports neurite extension. Neuron. 1991;7:209–220. - PubMed
-
- Chen H, Sedat JW, Agard DA. Manipulation, display and analysis of three dimensional biological images. In: Pawley J, editor. The Handbook of Biological Confocal Microscopy. IMR Press; Madison, Wisconsin: 1989. pp. 127–135.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

