Feeding a rich diet supplemented with the translation inhibitor cycloheximide decreases lifespan and ovary size in Drosophila
- PMID: 39588711
- PMCID: PMC11625892
- DOI: 10.1242/bio.061697
Feeding a rich diet supplemented with the translation inhibitor cycloheximide decreases lifespan and ovary size in Drosophila
Abstract
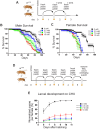
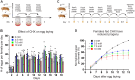
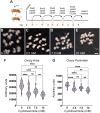
Drosophila oogenesis has long been an important model for understanding myriad cellular processes controlling development, RNA biology and patterning. Flies are easily fed drugs to disrupt various molecular pathways. However, this is often done under poor nutrient conditions that adversely affect oogenesis, thus making analysis challenging. Cycloheximide is a widely used compound that binds to and stalls the ribosome, therefore reducing protein synthesis. As egg production is a highly nutrient-dependent process, we developed a method to feed female Drosophila a rich diet of yeast paste supplemented with cycloheximide to better determine the effect of cycloheximide treatment on oogenesis. We found that flies readily consumed cycloheximide-supplemented yeast paste. Males and females had reduced lifespans when maintained on cycloheximide, with males exhibiting a dose-dependent decrease. Although females did not exhibit decreased egg laying, their ovaries were smaller and the number of progeny reduced, indicating substandard egg quality. Finally, females fed cycloheximide had disrupted oogenesis, with smaller ovaries, missing ovariole stages, and an increase in apoptotic follicles. Together, these data support that reduced protein synthesis adversely affects oogenesis with a rich diet that provides optimal nutrient conditions. In addition, this method could be used more broadly to test the effect of other drugs on Drosophila oogenesis without the confounding effects caused by poor nutrition.
Keywords: Drosophila; Cycloheximide; Lifespan; Nutrition; Oogenesis; Protein synthesis.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Update of
-
Feeding a rich diet supplemented with the translation inhibitor cycloheximide decreases lifespan and ovary size in Drosophila.bioRxiv [Preprint]. 2024 Aug 19:2024.08.19.608713. doi: 10.1101/2024.08.19.608713. bioRxiv. 2024. Update in: Biol Open. 2024 Nov 15;13(11):bio061697. doi: 10.1242/bio.061697. PMID: 39411156 Free PMC article. Updated. Preprint.
Similar articles
-
Feeding a rich diet supplemented with the translation inhibitor cycloheximide decreases lifespan and ovary size in Drosophila.bioRxiv [Preprint]. 2024 Aug 19:2024.08.19.608713. doi: 10.1101/2024.08.19.608713. bioRxiv. 2024. Update in: Biol Open. 2024 Nov 15;13(11):bio061697. doi: 10.1242/bio.061697. PMID: 39411156 Free PMC article. Updated. Preprint.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
References
-
- Belozerov, V. E., Ratkovic, S., Mcneill, H., Hilliker, A. J. and McDermott, J. C. (2014). In vivo interaction proteomics reveal a novel p38 mitogen-activated protein kinase/Rack1 pathway regulating proteostasis in Drosophila muscle. Mol. Cell. Biol. 34, 474-484. 10.1128/MCB.00824-13 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

