Ubiquitin-Mediated Effects on Oncogenesis during EBV and KSHV Infection
- PMID: 39459858
- PMCID: PMC11512223
- DOI: 10.3390/v16101523
Ubiquitin-Mediated Effects on Oncogenesis during EBV and KSHV Infection
Abstract
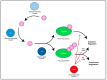
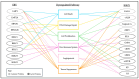
The Herpesviridae include the Epstein-Barr Virus (EBV) and the Kaposi Sarcoma-associated Herpesvirus (KSHV), both of which are oncogenic gamma-herpesviruses. These viruses manipulate host cellular mechanisms, including through ubiquitin-mediated pathways, to promote viral replication and oncogenesis. Ubiquitin, a regulatory protein which tags substrates for degradation or alters their function, is manipulated by both EBV and KSHV to facilitate viral persistence and cancer development. EBV infects approximately 90% of the global population and is implicated in malignancies including Burkitt lymphoma (BL), Hodgkin lymphoma (HL), post-transplant lymphoproliferative disorder (PTLD), and nasopharyngeal carcinoma. EBV latency proteins, notably LMP1 and EBNA3C, use ubiquitin-mediated mechanisms to inhibit apoptosis, promote cell proliferation, and interfere with DNA repair, contributing to tumorigenesis. EBV's lytic proteins, including BZLF1 and BPLF1, further disrupt cellular processes to favor oncogenesis. Similarly, KSHV, a causative agent of Kaposi's Sarcoma and lymphoproliferative disorders, has a latency-associated nuclear antigen (LANA) and other latency proteins that manipulate ubiquitin pathways to degrade tumor suppressors, stabilize oncogenic proteins, and evade immune responses. KSHV's lytic cycle proteins, such as RTA and Orf64, also use ubiquitin-mediated strategies to impair immune functions and promote oncogenesis. This review explores the ubiquitin-mediated interactions of EBV and KSHV proteins, elucidating their roles in viral oncogenesis. Understanding these mechanisms offers insights into the similarities between the viruses, as well as provoking thought about potential therapeutic targets for herpesvirus-associated cancers.
Keywords: EBV; Epstein–Barr Virus; KSHV; Kaposi Sarcoma-associated Herpesvirus; gammaherpesvirus; oncogenesis; post-translational modification; proteasomal degradation; tumor virus; ubiquitin.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Reduction of Kaposi's Sarcoma-Associated Herpesvirus Latency Using CRISPR-Cas9 To Edit the Latency-Associated Nuclear Antigen Gene.J Virol. 2019 Mar 21;93(7):e02183-18. doi: 10.1128/JVI.02183-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651362 Free PMC article.
-
EBV and KSHV Infection Dysregulates Autophagy to Optimize Viral Replication, Prevent Immune Recognition and Promote Tumorigenesis.Viruses. 2018 Oct 31;10(11):599. doi: 10.3390/v10110599. Viruses. 2018. PMID: 30384495 Free PMC article. Review.
-
Mutual inhibition between Kaposi's sarcoma-associated herpesvirus and Epstein-Barr virus lytic replication initiators in dually-infected primary effusion lymphoma.PLoS One. 2008 Feb 6;3(2):e1569. doi: 10.1371/journal.pone.0001569. PLoS One. 2008. PMID: 18253508 Free PMC article.
-
Cancers associated with human gammaherpesviruses.FEBS J. 2022 Dec;289(24):7631-7669. doi: 10.1111/febs.16206. Epub 2021 Oct 2. FEBS J. 2022. PMID: 34536980 Free PMC article. Review.
-
Manipulation of the host cell membrane by human γ-herpesviruses EBV and KSHV for pathogenesis.Virol Sin. 2016 Oct;31(5):395-405. doi: 10.1007/s12250-016-3817-2. Epub 2016 Sep 12. Virol Sin. 2016. PMID: 27624182 Free PMC article. Review.
References
-
- Whitley R.J. Herpesviruses. In: Baron S., editor. Medical Microbiology. 4th ed. University of Texas Medical Branch; Galveston, TX, USA: 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources