Predicting non-small cell lung cancer lymph node metastasis: integrating ctDNA mutation/methylation profiling with positron emission tomography-computed tomography (PET-CT) scan: protocol for a prospective clinical trial (LUNon-invasive Study)
- PMID: 39444874
- PMCID: PMC11494533
- DOI: 10.21037/jtd-24-1033
Predicting non-small cell lung cancer lymph node metastasis: integrating ctDNA mutation/methylation profiling with positron emission tomography-computed tomography (PET-CT) scan: protocol for a prospective clinical trial (LUNon-invasive Study)
Abstract
Background: Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases and remains a leading cause of cancer-related death. Lymph node metastasis (LNM) significantly affects recurrence, survival rates, and treatment options. While lymph node sampling is standard for surgically removing operable NSCLC, it can lead to complications. Positron emission tomography-computed tomography (PET-CT) helps assess preoperative LNM despite false positive or negative rates. Additionally, circulating tumor DNA (ctDNA) detects minimal residual disease with high sensitivity and specificity. Whether ctDNA can predict LNM in operable NSCLC remains uncertain. Our goal is to develop a precise model for predicting NSCLC LNM using non-invasive ctDNA/methylation profiling combined with PET-CT imaging.
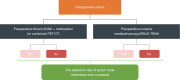
Methods: This is a prospective study conducted in three stages. We will enroll patients with clinical stage I-IIIB [8th tumor, node, metastasis (TNM) staging] NSCLC requiring lobectomy plus lymph node sampling/dissection. The distribution of clinical stages in the enrolled population is as follows: clinical stage cN0 (n=100) and cN1/cN2 (n=100). During Stage 1, we will establish LNMs-specific ctDNA methylation signatures and compare negative predictive value (NPV) rates of LNMs using preoperative blood ctDNA somatic mutation/methylation alone or combined with PET-CT across different groups. For Stage 2, we will compare detection rates between ctDNA somatic mutation/methylation profiles alone or combined with PET-CT and traditional mediastinoscopy/endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). As for Stage 3, ctDNA-free interval (CFI) and disease-free survival between systematic lymph node presence and absence in patients will be compared with preoperative negative ctDNA profiling and/or PET-CT. In Stage 3, patients will be followed up for 5 years to collect recurrence and survival data. Post-surgery follow-up ctDNA tests will be conducted every 3 months for the first 2 years, every 6 months for years 3-4, and annually in year five. Demographics and baseline data will be summarized with mean, standard deviation, median, max, and min values. Tests will include t-tests, Welch/Behren-Fisher test, and Wilcoxon rank-sum test for continuous variables. Categorical data will be presented as counts/percentages and compared using χ2 test or Fisher's exact test.
Discussion: By utilizing preoperative ctDNA/methylation profiling in conjunction with PET-CT, this study is expected to yield substantial evidence for accurately predicting LNM before surgery. This will help inform surgeons in selecting the appropriate intraoperative lymph node dissection strategy for operable NSCLC patients.
Trial registration: This study is registered on www.clinicaltrials.gov (NCT06358222).
Keywords: Non-small cell lung cancer (NSCLC); circulating tumor DNA (ctDNA); lymph node metastasis (LNM); methylation; positron emission tomography-computed tomography (PET-CT).
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1033/coif). F.Y. reports that the ctDNA test in this study was supported by the Liaoning Kanghui Biotechnology Co., Ltd. The other authors have no conflicts of interest to declare.
Figures



Similar articles
-
Positron emission tomography/computed tomography and endobronchial ultrasound-guided transbronchial needle aspiration to evaluate the status of N2 in preoperative non-small cell lung cancer: a diagnostic test.J Thorac Dis. 2022 Jun;14(6):2122-2130. doi: 10.21037/jtd-22-521. J Thorac Dis. 2022. PMID: 35813743 Free PMC article.
-
Impact of 18F-FDG PET/CT, CT and EBUS/TBNA on preoperative mediastinal nodal staging of NSCLC.BMC Med Imaging. 2021 Mar 17;21(1):49. doi: 10.1186/s12880-021-00580-w. BMC Med Imaging. 2021. PMID: 33731050 Free PMC article.
-
Prediction Models for Mediastinal Metastasis and Its Detection by Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Potentially Operable Non-Small Cell Lung Cancer: A Prospective Study.Chest. 2023 Sep;164(3):770-784. doi: 10.1016/j.chest.2023.03.041. Epub 2023 Apr 3. Chest. 2023. PMID: 37019355
-
ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer.Eur J Cardiothorac Surg. 2007 Jul;32(1):1-8. doi: 10.1016/j.ejcts.2007.01.075. Epub 2007 Apr 19. Eur J Cardiothorac Surg. 2007. PMID: 17448671
-
Lymph node assessment in early stage non-small cell lung cancer lymph node dissection or sampling?Gen Thorac Cardiovasc Surg. 2020 Jul;68(7):716-724. doi: 10.1007/s11748-020-01345-y. Epub 2020 Apr 7. Gen Thorac Cardiovasc Surg. 2020. PMID: 32266699 Review.
References
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
