CSF and blood levels of Neurofilaments, T-Tau, P-Tau, and Abeta-42 in amyotrophic lateral sclerosis: a systematic review and meta-analysis
- PMID: 39434139
- PMCID: PMC11492992
- DOI: 10.1186/s12967-024-05767-7
CSF and blood levels of Neurofilaments, T-Tau, P-Tau, and Abeta-42 in amyotrophic lateral sclerosis: a systematic review and meta-analysis
Abstract
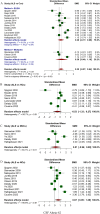
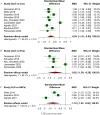
Recent literature suggests that markers of neuroaxonal damage, such as neurofilaments and tau protein, might serve as potential biomarkers for ALS. We conducted this systematic review and meta-analysis study to compare cerebrospinal fluid (CSF) and blood levels of total tau (t-tau), phosphorylated tau (p-tau), amyloid beta peptide 42 (Abeta-42), and neurofilaments in ALS patients and controls. A systematic search of Cochrane Library, PubMed, Embase, and ISI Web of Science was conducted on March 18, 2022, and updated on January 26, 2023. Observational studies that compared the concentrations of neurofilament light chain (NfL), neurofilament heavy chain (NFH), t-tau, p-tau, or Abeta-42 in CSF or peripheral blood of ALS patients and controls were included. Data from relevant studies were independently extracted and screened for quality using a standard tool, by at least two authors. Meta-analysis was conducted when a minimum of 3 studies reported the same biomarker within the same biofluid. A total of 100 studies were eligible for at least one meta-analysis. CSF and blood levels of NfL (standardized mean difference (SMD) [95% CI]; CSF: 1.46 [1.25-1.68]; blood: 1.35 [1.09-1.60]) and NFH (CSF: 1.32 [1.13-1.50], blood: 0.90 [0.58-1.22]) were significantly higher in ALS patients compared with controls. The pooled differences between ALS patients and controls were not significant for CSF t-tau, blood t-tau, and CSF Abeta-42, but CSF p-tau was lower in ALS patients (-0.27 [-0.47- -0.07]). Significantly decreased p-tau/t-tau ratios were found in ALS patients compared with controls (-0.84 [-1.16- -0.53]). Heterogeneity was considerable in most of our meta-analyses. CSF and blood neurofilament levels, as well as the CSF p-tau/t-tau ratio, might be potential candidates for improving ALS diagnosis. Further research is warranted to better understand the underlying mechanisms and the clinical implications of these biomarker alterations.
Keywords: ALS; Abeta-42; Alzheimer’s disease biomarkers; Amyotrophic lateral sclerosis; Biomarkers; Meta-analysis; Neurofilaments; Phosphorylated-tau; Systematic review; Total-tau.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Neurofilaments in Sporadic and Familial Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis.Genes (Basel). 2024 Apr 16;15(4):496. doi: 10.3390/genes15040496. Genes (Basel). 2024. PMID: 38674431 Free PMC article. Review.
-
CSF and blood biomarkers in amyotrophic lateral sclerosis: protocol for a systematic review and meta-analysis.Syst Rev. 2018 Dec 20;7(1):237. doi: 10.1186/s13643-018-0913-4. Syst Rev. 2018. PMID: 30572951 Free PMC article.
-
Diagnostic-prognostic value and electrophysiological correlates of CSF biomarkers of neurodegeneration and neuroinflammation in amyotrophic lateral sclerosis.J Neurol. 2020 Jun;267(6):1699-1708. doi: 10.1007/s00415-020-09761-z. Epub 2020 Feb 25. J Neurol. 2020. PMID: 32100123
-
Significance of CSF NfL and tau in ALS.J Neurol. 2018 Nov;265(11):2633-2645. doi: 10.1007/s00415-018-9043-0. Epub 2018 Sep 5. J Neurol. 2018. PMID: 30187162
-
Neurofilaments as Biomarkers for Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis.PLoS One. 2016 Oct 12;11(10):e0164625. doi: 10.1371/journal.pone.0164625. eCollection 2016. PLoS One. 2016. PMID: 27732645 Free PMC article. Review.
References
-
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis and other motor neuron disorders: official publication of the World Federation of Neurology. Res Group Motor Neuron Dis. 2000;1(5):293–9. 10.1080/146608200300079536. - PubMed
-
- Boekestein WA, Kleine BU, Hageman G, Schelhaas HJ, Zwarts MJ. (2010) Sensitivity and specificity of the ‘Awaji’ electrodiagnostic criteria for amyotrophic lateral sclerosis: retrospective comparison of the Awaji and revised El Escorial criteria for ALS. Amyotrophic lateral sclerosis: official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases 11 (6):497–501. 10.3109/17482961003777462 - PubMed
-
- Shefner JM, Al-Chalabi A, Baker MR, Cui LY, de Carvalho M, Eisen A, Grosskreutz J, Hardiman O, Henderson R, Matamala JM, Mitsumoto H, Paulus W, Simon N, Swash M, Talbot K, Turner MR, Ugawa Y, van den Berg LH, Verdugo R, Vucic S, Kaji R, Burke D, Kiernan MC. A proposal for new diagnostic criteria for ALS. Clin Neurophysiology: Official J Int Federation Clin Neurophysiol. 2020;131(8):1975–8. 10.1016/j.clinph.2020.04.005. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

