Five-year efficacy outcomes of ocrelizumab in relapsing multiple sclerosis: A propensity-matched comparison of the OPERA studies with other disease-modifying therapies in real-world lines of treatments
- PMID: 39290861
- PMCID: PMC11406495
- DOI: 10.1177/11795735241260563
Five-year efficacy outcomes of ocrelizumab in relapsing multiple sclerosis: A propensity-matched comparison of the OPERA studies with other disease-modifying therapies in real-world lines of treatments
Abstract
Background: Clinical trials comparing the efficacy of ocrelizumab (OCR) with other disease-modifying therapies (DMTs) other than interferon (IFN) β-1a in relapsing multiple sclerosis (RMS) are lacking.
Objectives: To compare the treatment effect of OCR vs six DMTs' (IFN β-1a, glatiramer acetate, fingolimod, dimethyl fumarate, teriflunomide, natalizumab) treatment pathways used in clinical practice by combining clinical trial and real-world data.
Methods: Patient-level data from OPERA trials and open-label extension phase, and from the German NeuroTransData (NTD) MS registry, were used to build 1:1 propensity score-matched (PSM) cohorts controlling for seven baseline covariates, including brain imaging activity. Efficacy outcomes were time to first relapse and time to 24-week confirmed disability progression over 5.5 years of follow-up. Intention-to-treat analysis using all outcome data irrespective of treatment switch was applied.
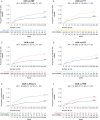
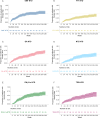
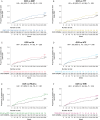
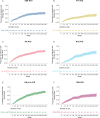
Results: The analyses included 611 OPERA patients and 7141 NTD patients. We built 12 paired-matched cohorts (six for each outcome, two for each DMT) to compare efficacy of OCR in OPERA with each DMT treatment pathway in NTD. Post-matching, baseline covariates and PS were well balanced (standardized mean difference <.2 for all cohorts). Over 5.5 years, patients treated with OCR showed a statistically significant reduction in the risk of relapse (hazard ratios [HRs] .30 to .54) and disability progression (HRs .51 to .67) compared with all index therapies and their treatment switching pathways in NTD. Treatment switch and/or discontinuation occurred frequently in NTD cohorts.
Conclusion: OCR demonstrates superiority in controlling relapses and disability progression in RMS compared with real-world treatment pathways over a 5.5-year period. These analyses suggest that high-efficacy DMTs and high treatment persistence are critical to achieve greatest clinical benefit in RMS.
Registration: OPERA I (NCT01247324), OPERA II (NCT01412333).
Keywords: Multiple sclerosis; comparative effectiveness research; ocrelizumab; real-world data; treatment pathways.
© The Author(s) 2024.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: F. Hoffmann-La Roche Ltd., Basel, Switzerland, provided financial support for the study and publication of this manuscript. Writing and editorial support was provided by Articulate Science, UK. E Muros-Le Rouzic is an employee of and shareholder in F. Hoffmann-La Roche Ltd. Y Heer was an employee of PricewaterhouseCoopers and contracted to perform statistical projects for NeuroTransData at the time of this analysis. S Yiu is an employee of Roche Products Ltd. V Tozzi is an employee of PricewaterhouseCoopers and contracted to perform statistical projects for NeuroTransData at the time of this analysis. S Braune has received honoraria from Kassenärztliche Vereinigung Bayerns and health maintenance organizations for patient care; and from Biogen, Merck, NeuroTransData, Novartis, and Roche for consulting, project management, clinical studies, and lectures; he also received honoraria and expense compensation as a board member of NeuroTransData. P van Hövell was an employee of PricewaterhouseCoopers and contracted to perform statistical projects for NeuroTransData at the time of this analysis. A Bergmann has received consultancy fees from advisory board, speaker, and other activities for NeuroTransData; project management and clinical studies for and travel expenses from Novartis and Servier. C Bernasconi is a consultant for F. Hoffmann-La Roche Ltd. F Model was an employee of and shareholder in F. Hoffmann-La Roche Ltd. at the time of this analysis and is now an employee of Denali Therapeutics. L Craveiro is an employee of and shareholder in F. Hoffmann-La Roche Ltd.
Figures

Similar articles
-
Real-world propensity score comparison of treatment effectiveness of peginterferon beta-1a vs. subcutaneous interferon beta-1a, glatiramer acetate, and teriflunomide in patients with relapsing-remitting multiple sclerosis.Mult Scler Relat Disord. 2021 Jun;51:102935. doi: 10.1016/j.msard.2021.102935. Epub 2021 Apr 8. Mult Scler Relat Disord. 2021. PMID: 33882426
-
Rituximab for people with multiple sclerosis.Cochrane Database Syst Rev. 2021 Nov 8;11(11):CD013874. doi: 10.1002/14651858.CD013874.pub2. Cochrane Database Syst Rev. 2021. PMID: 34748215 Free PMC article. Review.
-
Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials.JAMA Neurol. 2020 Sep 1;77(9):1132-1140. doi: 10.1001/jamaneurol.2020.1568. JAMA Neurol. 2020. PMID: 32511687 Free PMC article. Clinical Trial.
-
Work Productivity Outcomes Associated with Ocrelizumab Compared with Other Disease-Modifying Therapies for Multiple Sclerosis.Neurol Ther. 2021 Jun;10(1):183-196. doi: 10.1007/s40120-020-00224-1. Epub 2020 Nov 26. Neurol Ther. 2021. PMID: 33244713 Free PMC article.
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD011381. doi: 10.1002/14651858.CD011381.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174776 Free PMC article. Review.
References
-
- European Medicines Agency . Ocrevus [Summary of product characteristics], https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-.... (2021, accessed January 18, 2021). [Not Available in CrossRef].
-
- Genentech . Ocrevus (Ocrelizumab) [Full prescribing information], https://www.gene.com/download/pdf/ocrevus_prescribing.pdf. (2020, accessed January 20, 2020).
-
- Hauser SL Bar-Or A Comi G, et al. OPERA I and OPERA II Clinical Investigators. . Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221-234. - PubMed
-
- Bose D, Ravi R, Maurya M, Pushparajan L, Konwar M. Impact of disease-modifying therapies on MRI outcomes in patients with relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. Mult Scler Relat Disord. 2022;61:103760. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

