N otalgia P aresthetica De rmatologist R eport of Sy m ptom Burden and Treatment: Results from a Physician Survey
- PMID: 39262145
- PMCID: PMC11411784
- DOI: 10.2340/actadv.v104.39941
N otalgia P aresthetica De rmatologist R eport of Sy m ptom Burden and Treatment: Results from a Physician Survey
Abstract
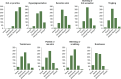
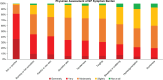
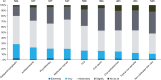
Notalgia paresthetica (NP) is a sensory neuropathy characterized by chronic pruritus, skin pain, and other pathologic sensations affecting the mid-to-upper back. NP may be under-recognized and under-diagnosed, with limited data available on its symptom presentation and treatment patterns. NP-DERM was an internet-based survey of dermatologists (n = 650) from 8 different countries on their perspectives on NP symptoms and current treatment practices. Dermatologists typically treated a median of 12 patients with NP per month. Dermatologists reported that itch (pruritus) was the most common symptom for their patients with NP, followed by hyperpigmentation and sensitive skin. The most burdensome NP symptom was pruritus, followed by burning or hot sensation, and painful or raw skin. The most prescribed treatments included non-medicated skin care, topical corticosteroids, oral antihistamines, medicated topicals, and gabapentin or pregabalin. Physicians reported low satisfaction with available treatments. The most common reason for physicians to discontinue patients' therapy was lack of response.
Conflict of interest statement
BSK: Founder – Klirna Biotech; consultant – 23andMe, ABRAX Japan, AbbVie, Almirall, Amagma Therapeutics, Amgen, Arcutis Biotherapeutics, Arena Pharmaceuticals, argenx, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Clexio Biosciences, Eli Lilly and Company, Escient Pharmaceuticals, Evommune, Galderma, Genentech, GlaxoSmith-Kline, Granular Therapeutics, Incyte Corporation, Innovaderm Research, Janssen, Kiniksa, LEO Pharma, Maruho, Novartis, Pfizer, Recens Medical, Regeneron Pharmaceuticals, Sanofi, Septerna, Vial, WebMD; stock in – ABRAX Japan, KliRNA Biotech, Locus Biosciences, and Recens Medical; patent for the use of JAK1 inhibitors for chronic pruritus; patent pending for the use of JAK inhibitors for interstitial cystitis; research grants – AbbVie, Cara Therapeutics, LEO Pharma, and Veradermics. SS: Speaker and/or consultant and/or investigator and/or has received research funding – AbbVie, Almirall, Beiersdorf, BMS, Clexio, Eli Lilly, FomF, Galderma, German Research Foundation (DFG), Integrity CE, Kiniksa, Leo Pharma, L’Oréal, MEDahead, Moroscience, NACCME, Novartis, Omnicuris, P.G. Unna Academy, Pfizer, Sanofi, TouchIME, UCB, Vifor, and WebMD. KK: Consulting fees and/or advisory board honoraria – Japan Tobacco Inc., Kao, LEO Pharma, Torii, Chugai Pharmaceutical, Maruho, Pola Pharma, AbbVie, Eli Lilly, Sanofi, and Pfizer; research grants – LEO Pharma, Japan Tobacco Inc., P&G Japan, Eli Lilly Japan, Tanabe Mitsubishi, Ono Pharmaceutical, Kyowa Kirin, Pola Pharma, AbbVie, Sanofi, Kose, Maruho, and Kyorin Pharmaceutical. NS, CM: Employment – Cara Therapeutics. JAM, SS, JG: Former employment – Cara Therapeutics. JCP: Former employment – AplusA Bell Falla, LLC. RA: Employment – AplusA Bell Falla, LLC. ML: Employment – Mount Sinai; research funding – AbbVie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Cara Therapeutics, Dermavant Sciences, Eli Lilly, Incyte, Inozyme, Janssen Research & Development, LLC, Novartis, Ortho Dermatologics, Regeneron, and UCB, Inc.; consultant - AnaptysBio, Arcutis, Inc., Arena Pharmaceuticals, Aristea Therapeutics, Avotres Therapeutics, BiomX, Boehringer Ingelheim, Brickell Biotech, Castle Biosciences, CorEvitas, Dermavant Sciences, Evommune, Inc., Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Hexima Ltd., Meiji Seika Pharma, Mindera, Pfizer, Seanergy, and Verrica.
Figures
Similar articles
-
Patient perspective on symptoms of Notalgia paresthetica: subpopulation results from the Neuropathic Itch Patient Survey (NIRVE).J Dermatolog Treat. 2024 Dec;35(1):2394107. doi: 10.1080/09546634.2024.2394107. Epub 2024 Aug 27. J Dermatolog Treat. 2024. PMID: 39191431
-
Successful treatment of notalgia paresthetica with lidocaine 5% medicated plaster: a case report.Pain Manag. 2022 Nov;12(8):887-894. doi: 10.2217/pmt-2022-0003. Epub 2022 Oct 3. Pain Manag. 2022. PMID: 36189717
-
NOTALGIA PARESTHETICA.Acta Clin Croat. 2018 Dec;57(4):721-725. doi: 10.20471/acc.2018.57.04.14. Acta Clin Croat. 2018. PMID: 31168209 Free PMC article. Review.
-
General features and treatment of notalgia paresthetica.Skinmed. 2011 Nov-Dec;9(6):353-8; quiz 359. Skinmed. 2011. PMID: 22256623 Review.
-
Notalgia paresthetica: clinical features, radiological evaluation, and a novel therapeutic option.BMC Neurol. 2020 May 16;20(1):191. doi: 10.1186/s12883-020-01773-6. BMC Neurol. 2020. PMID: 32416719 Free PMC article. Clinical Trial.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

