This is a preprint.
Molecular basis of convergent evolution of ACE2 receptor utilization among HKU5 coronaviruses
- PMID: 39253417
- PMCID: PMC11383307
- DOI: 10.1101/2024.08.28.608351
Molecular basis of convergent evolution of ACE2 receptor utilization among HKU5 coronaviruses
Abstract
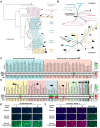
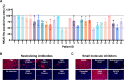
DPP4 was considered a canonical receptor for merbecoviruses until the recent discovery of African bat-borne MERS-related coronaviruses using ACE2. The extent and diversity with which merbecoviruses engage ACE2 and their receptor species tropism remain unknown. Here, we reveal that HKU5 enters host cells utilizing Pipistrellus abramus (P.abr) and several non-bat mammalian ACE2s through a binding mode distinct from that of any other known ACE2-using coronaviruses. These results show that several merbecovirus clades independently evolved ACE2 utilization, which appears to be a broadly shared property among these pathogens, through an extraordinary diversity of ACE2 recognition modes. We show that MERS-CoV and HKU5 have markedly distinct antigenicity, due to extensive genetic divergence, and identified several HKU5 inhibitors, including two clinical compounds. Our findings profoundly alter our understanding of coronavirus evolution and pave the way for developing countermeasures against viruses poised for human emergence.
Figures



Similar articles
-
ACE2 from Pipistrellus abramus bats is a receptor for HKU5 coronaviruses.bioRxiv [Preprint]. 2024 Aug 16:2024.03.13.584892. doi: 10.1101/2024.03.13.584892. bioRxiv. 2024. PMID: 38559009 Free PMC article. Preprint.
-
ACE2-using merbecoviruses: Further evidence of convergent evolution of ACE2 recognition by NeoCoV and other MERS-CoV related viruses.Cell Insight. 2024 Jan 30;3(1):100145. doi: 10.1016/j.cellin.2023.100145. eCollection 2024 Feb. Cell Insight. 2024. PMID: 38476250 Free PMC article. Review.
-
Replication of MERS and SARS coronaviruses in bat cells offers insights to their ancestral origins.Emerg Microbes Infect. 2018 Dec 10;7(1):209. doi: 10.1038/s41426-018-0208-9. Emerg Microbes Infect. 2018. PMID: 30531999 Free PMC article.
-
Genetic characterization of Betacoronavirus lineage C viruses in bats reveals marked sequence divergence in the spike protein of pipistrellus bat coronavirus HKU5 in Japanese pipistrelle: implications for the origin of the novel Middle East respiratory syndrome coronavirus.J Virol. 2013 Aug;87(15):8638-50. doi: 10.1128/JVI.01055-13. Epub 2013 May 29. J Virol. 2013. PMID: 23720729 Free PMC article.
-
[From SARS, MERS to COVID-19: A journey to understand bat coronaviruses].Bull Acad Natl Med. 2021 Aug;205(7):732-736. doi: 10.1016/j.banm.2021.05.008. Epub 2021 May 28. Bull Acad Natl Med. 2021. PMID: 34075253 Free PMC article. Review. French.
References
-
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., et al. (2003). Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1967–1976. - PubMed
-
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., et al. (2003). A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1953–1966. - PubMed
-
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., and Fouchier R.A. (2012). Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814–1820. - PubMed
-
- Mok C.K.P., Zhu A., Zhao J., Lau E.H.Y., Wang J., Chen Z., Zhuang Z., Wang Y., Alshukairi A.N., Baharoon S.A., et al. (2020). T-cell responses to MERS coronavirus infection in people with occupational exposure to dromedary camels in Nigeria: an observational cohort study. Lancet Infect. Dis. 10.1016/S1473-3099(20)30599-5. - DOI - PMC - PubMed
-
- Ngere I., Hunsperger E.A., Tong S., Oyugi J., Jaoko W., Harcourt J.L., Thornburg N.J., Oyas H., Muturi M., Osoro E.M., et al. (2022). Outbreak of Middle East Respiratory Syndrome Coronavirus in Camels and Probable Spillover Infection to Humans in Kenya. Viruses 14. 10.3390/v14081743. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
