A Targeted and Protease-Activated Genetically Encoded Melittin-Containing Particle for the Treatment of Cutaneous and Visceral Leishmaniasis
- PMID: 39240583
- PMCID: PMC11420870
- DOI: 10.1021/acsami.4c10426
A Targeted and Protease-Activated Genetically Encoded Melittin-Containing Particle for the Treatment of Cutaneous and Visceral Leishmaniasis
Abstract
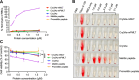
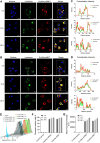
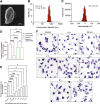
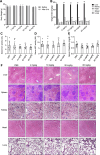
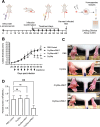
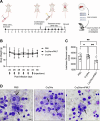
Intracellular infections are difficult to treat, as pathogens can take advantage of intracellular hiding, evade the immune system, and persist and multiply in host cells. One such intracellular parasite, Leishmania, is the causative agent of leishmaniasis, a neglected tropical disease (NTD), which disproportionately affects the world's most economically disadvantaged. Existing treatments have relied mostly on chemotherapeutic compounds that are becoming increasingly ineffective due to drug resistance, while the development of new therapeutics has been challenging due to the variety of clinical manifestations caused by different Leishmania species. The antimicrobial peptide melittin has been shown to be effective in vitro against a broad spectrum of Leishmania, including species that cause the most common form, cutaneous leishmaniasis, and the most deadly, visceral leishmaniasis. However, melittin's high hemolytic and cytotoxic activity toward host cells has limited its potential for clinical translation. Herein, we report a design strategy for producing a melittin-containing antileishmanial agent that not only enhances melittin's leishmanicidal potency but also abrogates its hemolytic and cytotoxic activity. This therapeutic construct can be directly produced in bacteria, significantly reducing its production cost critical for a NTD therapeutic. The designed melittin-containing fusion crystal incorporates a bioresponsive cathepsin linker that enables it to specifically release melittin in the phagolysosome of infected macrophages. Significantly, this targeted approach has been demonstrated to be efficacious in treating macrophages infected with L. amazonensis and L. donovani in cell-based models and in the corresponding cutaneous and visceral mouse models.
Keywords: Cry3Aa crystal protein; Leishmania; antileishmanial peptides; melittin; parasites.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
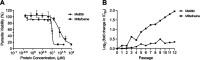

Similar articles
-
An effective in vitro and in vivo antileishmanial activity and mechanism of action of 8-hydroxyquinoline against Leishmania species causing visceral and tegumentary leishmaniasis.Vet Parasitol. 2016 Feb 15;217:81-8. doi: 10.1016/j.vetpar.2016.01.002. Epub 2016 Jan 7. Vet Parasitol. 2016. PMID: 26827866
-
Evaluation of the in vitro and in vivo antileishmanial activity of a chloroquinolin derivative against Leishmania species capable of causing tegumentary and visceral leishmaniasis.Exp Parasitol. 2019 Apr;199:30-37. doi: 10.1016/j.exppara.2019.02.019. Epub 2019 Feb 25. Exp Parasitol. 2019. PMID: 30817917
-
Efficacy of lapachol on treatment of cutaneous and visceral leishmaniasis.Exp Parasitol. 2019 Apr;199:67-73. doi: 10.1016/j.exppara.2019.02.013. Epub 2019 Feb 21. Exp Parasitol. 2019. PMID: 30797783
-
Exploring Innovative Leishmaniasis Treatment: Drug Targets from Pre-Clinical to Clinical Findings.Chem Biodivers. 2021 Sep;18(9):e2100336. doi: 10.1002/cbdv.202100336. Epub 2021 Aug 9. Chem Biodivers. 2021. PMID: 34369662 Review.
-
Miltefosine--discovery of the antileishmanial activity of phospholipid derivatives.Trans R Soc Trop Med Hyg. 2006 Dec;100 Suppl 1:S4-8. doi: 10.1016/j.trstmh.2006.03.009. Epub 2006 Aug 14. Trans R Soc Trop Med Hyg. 2006. PMID: 16904717 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

