This is a preprint.
Structural Study of Selectivity Mechanisms for JNK3 and p38α with Indazole Scaffold Probing Compounds
- PMID: 39149466
- PMCID: PMC11326381
- DOI: 10.21203/rs.3.rs-4730282/v1
Structural Study of Selectivity Mechanisms for JNK3 and p38α with Indazole Scaffold Probing Compounds
Abstract
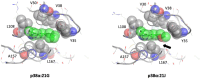
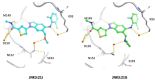
Selectivity is a primary focus in medicinal chemistry for ATP-competitive kinase inhibitors due to the highly conserved ATP binding pockets in the kinome. A decade of medicinal chemistry efforts has been carried out to develop selective inhibitors for JNKs, resulting in the identification of numerous promising scaffolds that even exhibit isoform selectivity. Thiophene-indazole is one of the scaffolds explored for isoform selectivity. Some iterations of this scaffold have also shown selectivity for p38α. In this study, we utilized four compounds derived from thiophene-indazole to investigate the mechanisms of selectivity for JNK3 and p38α. We determined crystal structures of the inhibitors bound to either JNK3 or p38α and subjected them to molecular dynamics (MD) simulations to understand the binding mechanism and critical interactions that govern affinity and selectivity for these two important kinases. The findings from this study provides valuable information for improving current lead inhibitors and developing a new generation of JNK3 isoform inhibitors.
Figures
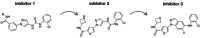
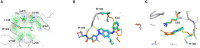
Similar articles
-
Discovery of Novel Indazole Chemotypes as Isoform-Selective JNK3 Inhibitors for the Treatment of Parkinson's Disease.J Med Chem. 2023 Jan 26;66(2):1273-1300. doi: 10.1021/acs.jmedchem.2c01410. Epub 2023 Jan 17. J Med Chem. 2023. PMID: 36649216
-
Structure-activity relationships and X-ray structures describing the selectivity of aminopyrazole inhibitors for c-Jun N-terminal kinase 3 (JNK3) over p38.J Biol Chem. 2009 May 8;284(19):12853-61. doi: 10.1074/jbc.M809430200. Epub 2009 Mar 4. J Biol Chem. 2009. PMID: 19261605 Free PMC article.
-
Thiophene-Pyrazolourea Derivatives as Potent, Orally Bioavailable, and Isoform-Selective JNK3 Inhibitors.ACS Med Chem Lett. 2020 Dec 13;12(1):24-29. doi: 10.1021/acsmedchemlett.0c00533. eCollection 2021 Jan 14. ACS Med Chem Lett. 2020. PMID: 33488960 Free PMC article.
-
A Comprehensive Structural Overview of p38α MAPK in Complex with Type I Inhibitors.ChemMedChem. 2015 Jun;10(6):957-69. doi: 10.1002/cmdc.201500030. Epub 2015 Apr 9. ChemMedChem. 2015. PMID: 26012502 Review.
-
A Perspective on the Development of c-Jun N-terminal Kinase Inhibitors as Therapeutics for Alzheimer's Disease: Investigating Structure through Docking Studies.Biomedicines. 2021 Oct 9;9(10):1431. doi: 10.3390/biomedicines9101431. Biomedicines. 2021. PMID: 34680547 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

