Extracellular Vesicles as Mediators of Neuroinflammation in Intercellular and Inter-Organ Crosstalk
- PMID: 39000150
- PMCID: PMC11241119
- DOI: 10.3390/ijms25137041
Extracellular Vesicles as Mediators of Neuroinflammation in Intercellular and Inter-Organ Crosstalk
Abstract
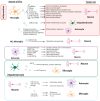
Neuroinflammation, crucial in neurological disorders like Alzheimer's disease, multiple sclerosis, and hepatic encephalopathy, involves complex immune responses. Extracellular vesicles (EVs) play a pivotal role in intercellular and inter-organ communication, influencing disease progression. EVs serve as key mediators in the immune system, containing molecules capable of activating molecular pathways that exacerbate neuroinflammatory processes in neurological disorders. However, EVs from mesenchymal stem cells show promise in reducing neuroinflammation and cognitive deficits. EVs can cross CNS barriers, and peripheral immune signals can influence brain function via EV-mediated communication, impacting barrier function and neuroinflammatory responses. Understanding EV interactions within the brain and other organs could unveil novel therapeutic targets for neurological disorders.
Keywords: CNS barrier; exosomes; extracellular vesicles; glial cells; inter-organ crosstalk; neuroinflammation; neurological disorders; neuron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Brain incoming call from glia during neuroinflammation: Roles of extracellular vesicles.Neurobiol Dis. 2024 Oct 15;201:106663. doi: 10.1016/j.nbd.2024.106663. Epub 2024 Sep 7. Neurobiol Dis. 2024. PMID: 39251030 Review.
-
Extracellular vesicles in the treatment of neurological disorders.Neurobiol Dis. 2021 Sep;157:105445. doi: 10.1016/j.nbd.2021.105445. Epub 2021 Jul 14. Neurobiol Dis. 2021. PMID: 34271084 Free PMC article. Review.
-
Characterization of spinal cord tissue-derived extracellular vesicles in neuroinflammation.J Neuroinflammation. 2024 Jun 8;21(1):154. doi: 10.1186/s12974-024-03147-y. J Neuroinflammation. 2024. PMID: 38851724 Free PMC article.
-
Emerging Role of Extracellular Vesicles in Intercellular Communication in the Brain: Implications for Neurodegenerative Diseases and Therapeutics.Cell Biochem Biophys. 2024 Jun;82(2):379-398. doi: 10.1007/s12013-024-01221-z. Epub 2024 Feb 1. Cell Biochem Biophys. 2024. PMID: 38300375 Review.
-
Looking to the Future of the Role of Macrophages and Extracellular Vesicles in Neuroinflammation in ALS.Int J Mol Sci. 2023 Jul 8;24(14):11251. doi: 10.3390/ijms241411251. Int J Mol Sci. 2023. PMID: 37511010 Free PMC article. Review.
Cited by
-
Emerging Role of Extracellular Vesicles as Biomarkers in Neurodegenerative Diseases and Their Clinical and Therapeutic Potential in Central Nervous System Pathologies.Int J Mol Sci. 2024 Sep 19;25(18):10068. doi: 10.3390/ijms251810068. Int J Mol Sci. 2024. PMID: 39337560 Free PMC article. Review.
-
Unveiling the multifaceted roles of microRNAs in extracellular vesicles derived from mesenchymal stem cells: implications in tumor progression and therapeutic interventions.Front Pharmacol. 2024 Aug 5;15:1438177. doi: 10.3389/fphar.2024.1438177. eCollection 2024. Front Pharmacol. 2024. PMID: 39161894 Free PMC article. Review.
References
-
- Cabrera-Pastor A., Llansola M., Montoliu C., Malaguarnera M., Balzano T., Taoro-Gonzalez L., García-García R., Mangas-Losada A., Izquierdo-Altarejos P., Arenas Y.M., et al. Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: Underlying mechanisms and therapeutic implications. Acta Physiol. 2019;226:e13270. doi: 10.1111/apha.13270. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

