NADPH oxidase 2 activity disrupts Calmodulin/CaMKIIα complex via redox modifications of CaMKIIα-contained Cys30 and Cys289: Implications in Parkinson's disease
- PMID: 38968922
- PMCID: PMC11278932
- DOI: 10.1016/j.redox.2024.103254
NADPH oxidase 2 activity disrupts Calmodulin/CaMKIIα complex via redox modifications of CaMKIIα-contained Cys30 and Cys289: Implications in Parkinson's disease
Abstract
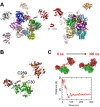
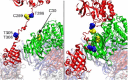
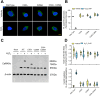
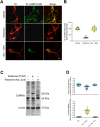
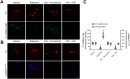
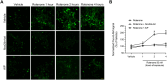
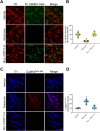
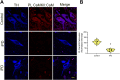
Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα) signaling in the brain plays a critical role in regulating neuronal Ca2+ homeostasis. Its dysfunctional activity is associated with various neurological and neurodegenerative disorders, including Parkinson's disease (PD). Using computational modeling analysis, we predicted that, two essential cysteine residues contained in CaMKIIα, Cys30 and Cys289, may undergo redox modifications impacting the proper functioning of the CaMKIIα docking site for Ca2+/CaM, thus impeding the formation of the CaMKIIα:Ca2+/CaM complex, essential for a proper modulation of CaMKIIα kinase activity. Our subsequent in vitro investigations confirmed the computational predictions, specifically implicating Cys30 and Cys289 residues in impairing CaMKIIα:Ca2+/CaM interaction. We observed CaMKIIα:Ca2+/CaM complex disruption in dopamine (DA) nigrostriatal neurons of post-mortem Parkinson's disease (PD) patients' specimens, addressing the high relevance of this event in the disease. CaMKIIα:Ca2+/CaM complex disruption was also observed in both in vitro and in vivo rotenone models of PD, where this phenomenon was associated with CaMKIIα kinase hyperactivity. Moreover, we observed that, NADPH oxidase 2 (NOX2), a major enzymatic generator of superoxide anion (O2●-) and hydrogen peroxide (H2O2) in the brain with implications in PD pathogenesis, is responsible for CaMKIIα:Ca2+/CaM complex disruption associated to a stable Ca2+CAM-independent CaMKIIα kinase activity and intracellular Ca2+ accumulation. The present study highlights the importance of oxidative stress, in disturbing the delicate balance of CaMKIIα signaling in calcium dysregulation, offering novel insights into PD pathogenesis.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The Authors declare no conflicts of interest.
Figures

Similar articles
-
Mechanisms of Ca2+/calmodulin-dependent kinase II activation in single dendritic spines.Nat Commun. 2019 Jun 25;10(1):2784. doi: 10.1038/s41467-019-10694-z. Nat Commun. 2019. PMID: 31239443 Free PMC article.
-
Redox-sensitive calcium/calmodulin-dependent protein kinase IIα in angiotensin II intra-neuronal signaling and hypertension.Redox Biol. 2019 Oct;27:101230. doi: 10.1016/j.redox.2019.101230. Epub 2019 May 30. Redox Biol. 2019. PMID: 31175066 Free PMC article.
-
Interactions of CaMKII with dopamine D2 receptors: roles in levodopa-induced dyskinesia in 6-hydroxydopamine lesioned Parkinson's rats.Sci Rep. 2014 Oct 29;4:6811. doi: 10.1038/srep06811. Sci Rep. 2014. PMID: 25351365 Free PMC article.
-
Stimulation-induced changes in diffusion and structure of calmodulin and calmodulin-dependent protein kinase II proteins in neurons.Neurosci Res. 2018 Nov;136:13-32. doi: 10.1016/j.neures.2018.01.003. Epub 2018 Feb 1. Neurosci Res. 2018. PMID: 29395358 Review.
-
Calmodulin and Its Binding Proteins in Parkinson's Disease.Int J Mol Sci. 2021 Mar 16;22(6):3016. doi: 10.3390/ijms22063016. Int J Mol Sci. 2021. PMID: 33809535 Free PMC article. Review.
References
-
- Rotenberg A., et al. Mice expressing activated CaMKII lack low frequency LTP and do not form stable place cells in the CA1 region of the hippocampus. Cell. 1996;87(7):1351–1361. - PubMed
-
- Baudat F., et al. [Prdm9, a key control of mammalian recombination hotspots] Med. Sci. 2010;26(5):468–470. - PubMed
-
- Itagaki C., et al. Stimulus-coupled interaction of tyrosine hydroxylase with 14-3-3 proteins. Biochemistry. 1999;38(47):15673–15680. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

