Seven robust and easy to obtain biomarkers to measure acute stress
- PMID: 38799794
- PMCID: PMC11126813
- DOI: 10.1016/j.bbih.2024.100789
Seven robust and easy to obtain biomarkers to measure acute stress
Abstract
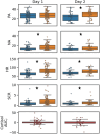
With the purpose of identifying a sensitive, robust, and easy-to-measure set of biomarkers to assess stress reactivity, we here study a large set of relatively easy to obtain markers reflecting subjective, autonomic nervous system (ANS), endocrine, and inflammatory responses to acute social stress (n = 101). A subset of the participants was exposed to another social stressor the next day (n = 48) while being measured in the same way. Acute social stress was induced following standardized procedures. The markers investigated were self-reported positive and negative affect, heart rate, electrodermal activity, salivary cortisol, and ten inflammatory markers both in capillary plasma and salivary samples, including IL-22 which has not been studied in response to acute stress in humans before. Robust effects (significant effect in the same direction for both days) were found for self-reported negative affect, heart rate, electrodermal activity, plasma IL-5, plasma IL-22, salivary IL-8 and salivary IL-10. Of these seven markers, the participants' IL-22 responses on the first day were positively correlated to those on the second day. We found no correlations between salivary and capillary plasma stress responses for any of the ten cytokines and somewhat unexpectedly, cytokine responses in saliva seemed more pronounced and more in line with previous literature than cytokines in capillary plasma. In sum, seven robust and easy to obtain biomarkers to measure acute stress response were identified and should be used in future stress research to detect and examine stress reactivity. This includes IL-22 in plasma as a promising novel marker.
Keywords: Acute stress response; Cortisol; Cytokines; Immune; Multimodal; Psychophysiology.
© 2024 The Authors.
Conflict of interest statement
The authors report there are no competing interests to declare.
Figures
Similar articles
-
Salivary markers of inflammation in response to acute stress.Brain Behav Immun. 2015 Feb;44:253-69. doi: 10.1016/j.bbi.2014.08.008. Epub 2014 Sep 7. Brain Behav Immun. 2015. PMID: 25205395 Free PMC article. Review.
-
Better cognitive control of emotional information is associated with reduced pro-inflammatory cytokine reactivity to emotional stress.Stress. 2016;19(1):63-8. doi: 10.3109/10253890.2015.1121983. Epub 2016 Jan 13. Stress. 2016. PMID: 26581830 Free PMC article.
-
HPA-axis and inflammatory reactivity to acute stress is related with basal HPA-axis activity.Psychoneuroendocrinology. 2017 Apr;78:168-176. doi: 10.1016/j.psyneuen.2017.01.035. Epub 2017 Feb 6. Psychoneuroendocrinology. 2017. PMID: 28209543 Free PMC article.
-
Acute Dental Pain and Salivary Biomarkers for Stress and Inflammation in Patients with Pulpal or Periapical Inflammation.J Oral Facial Pain Headache. 2019 Spring;33(2):227–233. doi: 10.11607/ofph.2007. Epub 2018 Oct 10. J Oral Facial Pain Headache. 2019. PMID: 30304079
-
Cortisol reactivity in social anxiety disorder: A highly standardized and controlled study.Psychoneuroendocrinology. 2021 Jan;123:104913. doi: 10.1016/j.psyneuen.2020.104913. Epub 2020 Oct 15. Psychoneuroendocrinology. 2021. PMID: 33160230
References
-
- Allen A.P., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014;38:94–124. - PubMed
-
- Andoh A., Zhang Z., Inatomi O., Fujino S., Deguchi Y., Araki Y., Tsujikawa T., Kitoh K., Kim-Mitsuyama S., Takayanagi A., Shimizu N., Fujiyama Y. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129(3):969–984. doi: 10.1053/j.gastro.2005.06.071. Sep. PMID: 16143135. - DOI - PubMed
LinkOut - more resources
Full Text Sources

