Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
- PMID: 38462638
- PMCID: PMC10925609
- DOI: 10.1038/s41392-024-01771-x
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Abstract
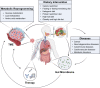
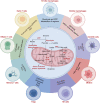
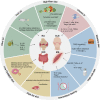
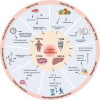
Diet, serving as a vital source of nutrients, exerts a profound influence on human health and disease progression. Recently, dietary interventions have emerged as promising adjunctive treatment strategies not only for cancer but also for neurodegenerative diseases, autoimmune diseases, cardiovascular diseases, and metabolic disorders. These interventions have demonstrated substantial potential in modulating metabolism, disease trajectory, and therapeutic responses. Metabolic reprogramming is a hallmark of malignant progression, and a deeper understanding of this phenomenon in tumors and its effects on immune regulation is a significant challenge that impedes cancer eradication. Dietary intake, as a key environmental factor, can influence tumor metabolism. Emerging evidence indicates that dietary interventions might affect the nutrient availability in tumors, thereby increasing the efficacy of cancer treatments. However, the intricate interplay between dietary interventions and the pathogenesis of cancer and other diseases is complex. Despite encouraging results, the mechanisms underlying diet-based therapeutic strategies remain largely unexplored, often resulting in underutilization in disease management. In this review, we aim to illuminate the potential effects of various dietary interventions, including calorie restriction, fasting-mimicking diet, ketogenic diet, protein restriction diet, high-salt diet, high-fat diet, and high-fiber diet, on cancer and the aforementioned diseases. We explore the multifaceted impacts of these dietary interventions, encompassing their immunomodulatory effects, other biological impacts, and underlying molecular mechanisms. This review offers valuable insights into the potential application of these dietary interventions as adjunctive therapies in disease management.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Intermittent fasting interventions for the treatment of overweight and obesity in adults aged 18 years and over: a systematic review protocol.JBI Database System Rev Implement Rep. 2015 Oct;13(10):60-8. doi: 10.11124/jbisrir-2015-2363. JBI Database System Rev Implement Rep. 2015. PMID: 26571283
-
Caloric restriction for the management of malignant tumors - from animal studies towards clinical translation.Int J Vitam Nutr Res. 2024 Feb;94(1):1-9. doi: 10.1024/0300-9831/a000779. Epub 2023 Feb 9. Int J Vitam Nutr Res. 2024. PMID: 36755497
-
Dietary approaches for exploiting metabolic vulnerabilities in cancer.Biochim Biophys Acta Rev Cancer. 2024 Mar;1879(2):189062. doi: 10.1016/j.bbcan.2023.189062. Epub 2023 Dec 28. Biochim Biophys Acta Rev Cancer. 2024. PMID: 38158024 Review.
-
Advances in Diet and Physical Activity in Breast Cancer Prevention and Treatment.Nutrients. 2024 Jul 13;16(14):2262. doi: 10.3390/nu16142262. Nutrients. 2024. PMID: 39064705 Free PMC article. Review.
-
Current advances in cancer energy metabolism under dietary restriction: a mini review.Med Oncol. 2024 Jul 26;41(9):209. doi: 10.1007/s12032-024-02452-z. Med Oncol. 2024. PMID: 39060824 Review.
Cited by
-
Antibiotic-induced gut microbiota disruption promotes vascular calcification by reducing short-chain fatty acid acetate.Mol Med. 2024 Aug 24;30(1):130. doi: 10.1186/s10020-024-00900-0. Mol Med. 2024. PMID: 39182021 Free PMC article.
-
Caloric restriction impacts skin barrier function and attenuates the development of hyperplasia skin disease.Front Nutr. 2024 Sep 20;11:1423524. doi: 10.3389/fnut.2024.1423524. eCollection 2024. Front Nutr. 2024. PMID: 39371941 Free PMC article.
-
Does tinnitus amplify the effects of healthy eating patterns and physical activity on the sleep disturbance or sleep insufficiency, based on the case study of NHANES survey in the United States.Front Nutr. 2024 Aug 29;11:1427672. doi: 10.3389/fnut.2024.1427672. eCollection 2024. Front Nutr. 2024. PMID: 39267856 Free PMC article.
-
Dietary Flavonoids: Mitigating Air Pollution's Cardiovascular Risks.Nutrients. 2024 Aug 10;16(16):2647. doi: 10.3390/nu16162647. Nutrients. 2024. PMID: 39203784 Free PMC article. Review.
-
Integrated multi-omics profiling highlights the diet-gut-brain axis in low-calorie diets promoted novelty-seeking behavior.Curr Res Food Sci. 2024 Oct 28;9:100897. doi: 10.1016/j.crfs.2024.100897. eCollection 2024. Curr Res Food Sci. 2024. PMID: 39555017 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

