Efficacy of empagliflozin as adjunctive therapy to citalopram in major depressive disorder: a randomized double-blind, placebo-controlled clinical trial
- PMID: 38408937
- PMCID: PMC10895773
- DOI: 10.1186/s12888-024-05627-0
Efficacy of empagliflozin as adjunctive therapy to citalopram in major depressive disorder: a randomized double-blind, placebo-controlled clinical trial
Abstract
Background: Major depressive disorder is one of the most common psychiatric disorders, which is associated with a high disease burden. Current treatments using antidepressants have limitations, so using medication with neuromodulating and anti-inflammatory properties alongside them could be helpful. In a clinical trial, we studied the effectiveness of empagliflozin, a blood sugar-lowering drug, as an adjunctive therapy to reduce the severity of depression symptoms.
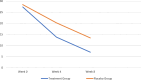
Methods: A number of outpatients with moderate to severe depression (Hamilton Depression Rating Scale (HDRS) > = 17) who were not under related medication or had not taken medication for at least the last two months, had an age range of 18-60 years and had written informed consent to enter the study (N = 90) were randomly divided into two groups receiving placebo or empagliflozin (10 mg daily) combined with citalopram (40 mg daily) based on permuted block randomization method in an 8-week randomized, double-blind, placebo-controlled clinical trial. They were evaluated using the HDRS in weeks 0, 4, and 8.
Results: HDRS scores were equal to 28.42(± 3.83), 20.20(± 3.82), and 13.42(± 3.42) in the placebo group during weeks 0,4, and 8, respectively. These scores were 27.36(± 3.77), 13.76(± 1.40), and 7.00(± 1.13), respectively, for the group treated with empagliflozin. Compared to the control group, patients treated with empagliflozin using repeated-measures ANOVA showed greater improvement in reducing the severity of depression symptoms over time (p value = 0.0001).
Conclusions: Considering the promising findings in this clinical trial, further study of empagliflozin as adjunctive therapy in MDD with larger sample sizes and longer follow-ups is recommended.
Keywords: Anti-inflammatory; Citalopram; Empagliflozin; Major depressive disorder; Neuromodulating.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
-
Lamotrigine versus levetiracetam or zonisamide for focal epilepsy and valproate versus levetiracetam for generalised and unclassified epilepsy: two SANAD II non-inferiority RCTs.Health Technol Assess. 2021 Dec;25(75):1-134. doi: 10.3310/hta25750. Health Technol Assess. 2021. PMID: 34931602 Clinical Trial.
-
Mobile apps to reduce depressive symptoms and alcohol use in youth: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 26;20(2):e1398. doi: 10.1002/cl2.1398. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38680950 Free PMC article. Review.
-
Comparison of cognitive behaviour therapy versus activity management, both delivered remotely, to treat paediatric chronic fatigue syndrome/myalgic encephalomyelitis: the UK FITNET-NHS RCT.Health Technol Assess. 2024 Oct;28(70):1-134. doi: 10.3310/VLRW6701. Health Technol Assess. 2024. PMID: 39485730 Free PMC article. Clinical Trial.
Cited by
-
Sodium-glucose cotransporter 1/2 inhibition and risk of neurodegenerative disorders: A Mendelian randomization study.Brain Behav. 2024 Jul;14(7):e3624. doi: 10.1002/brb3.3624. Brain Behav. 2024. PMID: 39010704 Free PMC article.
References
-
- Ruhe HG, Mocking RJ, Figueroa CA, Seeverens PW, Ikani N, Tyborowska A, et al. Emotional biases and recurrence in major depressive disorder. Results of 2.5 years follow-up of drug-free cohort vulnerable for recurrence. Front Psychiatry. 2019;10:145. doi: 10.3389/fpsyt.2019.00145. - DOI - PMC - PubMed
-
- Harris MG, Kazdin AE, Chiu WT, Sampson NA, Aguilar-Gaxiola S, Al-Hamzawi A, et al. Findings from world mental health surveys of the perceived helpfulness of treatment for patients with major depressive disorder. JAMA Psychiat. 2020;77(8):830–841. doi: 10.1001/jamapsychiatry.2020.1107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources