Angiotensin II induces endothelial dysfunction and vascular remodeling by downregulating TRPV4 channels
- PMID: 38333200
- PMCID: PMC10852140
- DOI: 10.1016/j.jmccpl.2023.100055
Angiotensin II induces endothelial dysfunction and vascular remodeling by downregulating TRPV4 channels
Abstract
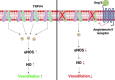
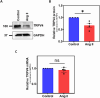
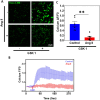
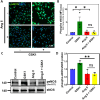
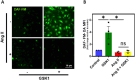
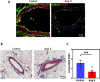
Angiotensin II (Ang II) is a potent vasoconstrictor of vascular smooth muscle cells (VSMC) and is implicated in hypertension, but it's role in the regulation of endothelial function is not well known. We and others have previously shown that mechanically activated ion channel, Transient Receptor Potential Vanilloid 4 (TRPV4) mediates flow- and/or receptor-dependent vasodilation via nitric oxide (NO) production in endothelial cells. Ang II was demonstrated to crosstalk with TRPV4 via angiotensin 1 receptor (AT1R) and β-arrestin signaling in epithelial and immortalized cells, however, the role of this crosstalk in endothelial cell function is not fully explored. Ang II treatment significantly downregulated TRPV4 protein expression and TRPV4-mediated Ca2+ influx in human EC without altering TRPV4 mRNA levels. Further, TRPV4-induced eNOS phosphorylation and NO production were significantly reduced in Ang II-treated human EC. Importantly, Ang II infusion in mice revealed that, TRPV4/p-eNOS expression and colocalization was reduced in endothelium in vivo. Finally, Ang II infusion induced vascular remodeling as evidenced by decreased lumen to wall ratio in resistant mesenteric arteries. These findings suggest that Ang II induces endothelial dysfunction and vascular remodeling via downregulation of TRPV4/eNOS pathway and may contribute to hypertension, independent of or in addition to its effect on vascular smooth muscle contraction.
Keywords: Angiotensin II; Ca2+; Endothelial cells; TRPV4; Vascular remodeling; eNOS.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Myoendothelial feedback in mouse mesenteric resistance arteries is similar between the sexes, dependent on nitric oxide synthase, and independent of TPRV4.Am J Physiol Heart Circ Physiol. 2024 Jan 1;326(1):H190-H202. doi: 10.1152/ajpheart.00170.2023. Epub 2023 Nov 3. Am J Physiol Heart Circ Physiol. 2024. PMID: 37921665 Free PMC article.
-
Angiotensin II Induces Vascular Endothelial Dysfunction by Promoting Lipid Peroxidation-Mediated Ferroptosis via CD36.Biomolecules. 2024 Nov 17;14(11):1456. doi: 10.3390/biom14111456. Biomolecules. 2024. PMID: 39595632 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Ang-(1-7) and ET-1 Interplay Through Mas and ETB Receptor Interaction Defines a Novel Vasoprotective Mechanism.Hypertension. 2025 Feb;82(2):267-281. doi: 10.1161/HYPERTENSIONAHA.124.22693. Epub 2024 Dec 5. Hypertension. 2025. PMID: 39633565
-
Drug therapy for obstructive sleep apnoea in adults.Cochrane Database Syst Rev. 2013 May 31;2013(5):CD003002. doi: 10.1002/14651858.CD003002.pub3. Cochrane Database Syst Rev. 2013. PMID: 23728641 Free PMC article. Review.
Cited by
-
TRPV4-A Multifunctional Cellular Sensor Protein with Therapeutic Potential.Sensors (Basel). 2024 Oct 29;24(21):6923. doi: 10.3390/s24216923. Sensors (Basel). 2024. PMID: 39517820 Free PMC article. Review.
-
Sex differences and role of lysyl oxidase-like 2 in angiotensin II-induced hypertension in mice.Am J Physiol Heart Circ Physiol. 2024 Sep 1;327(3):H642-H659. doi: 10.1152/ajpheart.00110.2024. Epub 2024 Jul 19. Am J Physiol Heart Circ Physiol. 2024. PMID: 39028284
References
-
- Tackling G., Borhade M.B. StatPearls; Treasure Island (FL): 2023. Hypertensive heart disease.
-
- Lu Y., Sun X., Peng L., Jiang W., Li W., Yuan H., et al. Angiotensin II-induced vascular remodeling and hypertension involves cathepsin L/V- MEK/ERK mediated mechanism. Int J Cardiol. 2020;298:98–106. - PubMed
-
- Schiffrin E.L. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension. 2012;59(2):367–374. - PubMed
-
- Griendling K.K., Ushio-Fukai M., Lassegue B., Alexander R.W. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension. 1997;29(1 Pt 2):366–373. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

