Effects of glucocorticoid use on survival of advanced non-small-cell lung cancer patients treated with immune checkpoint inhibitors
- PMID: 37925595
- PMCID: PMC10617908
- DOI: 10.1097/CM9.0000000000002544
Effects of glucocorticoid use on survival of advanced non-small-cell lung cancer patients treated with immune checkpoint inhibitors
Abstract
Background: Lung cancer is the second most common cancer worldwide, with non-small-cell lung cancer (NSCLC) accounting for the majority of cases. Patients with NSCLC have achieved great survival benefits from immunotherapies targeting immune checkpoints. Glucocorticoids (GCs) are frequently used for palliation of cancer-associated symptoms, as supportive care for non-cancer-associated symptoms, and for management of immune-related adverse events (irAEs). The aim of this study was to clarify the safety and prognostic significance of glucocorticoid use in advanced patients with NSCLC treated with immune checkpoint inhibitors (ICIs).
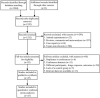
Methods: The study searched publications from PubMed, Embase, Cochrane Library, Web of Science, China Biology Medicine disc, Chinese National Knowledge Infrastructure, Wanfang Data, and Chinese Science and Technology Journal Database up to March 1st, 2022, and conducted a meta-analysis to assess the effects of glucocorticoid use on overall survival (OS) and progression-free survival (PFS) in NSCLC patients treated with ICIs through the available data. The study calculated the pooled hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: This study included data from 25 literatures that were mainly retrospective, with 8713 patients included. Patients taking GCs had a higher risk for tumor progression and death compared with those not taking GCs (PFS: HR = 1.57, 95% CI: 1.33-1.86, P <0.001; OS: HR = 1.63, 95% CI: 1.41-1.88, P <0.001). GCs used for cancer-associated symptoms caused an obviously negative effect on both PFS and OS (PFS: HR = 1.74, 95% CI: 1.32-2.29, P <0.001; OS: HR = 1.76, 95% CI: 1.52-2.04, P <0.001). However, GCs used for irAEs management did not negatively affect prognosis (PFS: HR = 0.68, 95% CI: 0.46-1.00, P = 0.050; OS: HR = 0.53, 95% CI: 0.34-0.83, P = 0.005), and GCs used for non-cancer-associated indications had no effect on prognosis (PFS: HR = 0.92, 95%CI: 0.63-1.32, P = 0.640; OS: HR = 0.91, 95% CI: 0.59-1.41, P = 0.680).
Conclusions: In advanced NSCLC patients treated with ICIs, the use of GCs for palliation of cancer-associated symptoms may result in a worse PFS and OS, indicating that they increase the risk of tumor progression and death. But, in NSCLC patients treated with ICIs, the use of GCs for the management of irAEs may be safe, and the use of GCs for the treatment of non-cancer-associated symptoms may not affect the ICIs' survival benefits. Therefore, it is necessary to be careful and evaluate indications rationally before administering GCs in individualized clinical management.
Copyright © 2023 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures



Similar articles
-
Corticosteroid administration for cancer-related indications is an unfavorable prognostic factor in solid cancer patients receiving immune checkpoint inhibitor treatment.Int Immunopharmacol. 2021 Oct;99:108031. doi: 10.1016/j.intimp.2021.108031. Epub 2021 Aug 3. Int Immunopharmacol. 2021. PMID: 34358857
-
How to Choose a Survival Period? The Impact of Antibiotic Use on OS or PFS in NSCLC Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis.Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211033498. doi: 10.1177/15330338211033498. Technol Cancer Res Treat. 2021. PMID: 34323149 Free PMC article.
-
Patients with melanoma treated with immune checkpoint inhibitors who had non-thyroid endocrine and skin immune-related adverse events have better prognosis: A systematic review and meta-analysis.Front Oncol. 2022 Sep 14;12:976224. doi: 10.3389/fonc.2022.976224. eCollection 2022. Front Oncol. 2022. PMID: 36185176 Free PMC article.
-
Efficacy of PD-1/PD-L1 Inhibitors versus Chemotherapy in Lung Cancer with Brain Metastases: A Systematic Review and Meta-Analysis.J Immunol Res. 2022 May 20;2022:4518898. doi: 10.1155/2022/4518898. eCollection 2022. J Immunol Res. 2022. PMID: 35637793 Free PMC article. Review.
-
Immune checkpoint inhibitors' combination therapy as first-line treatment in advanced esophageal squamous cell carcinoma: a meta-analysis.J Cancer Res Clin Oncol. 2023 Mar;149(3):933-939. doi: 10.1007/s00432-022-04066-2. Epub 2022 Jun 25. J Cancer Res Clin Oncol. 2023. PMID: 35751682 Review.
Cited by
-
Strategies to enhance the therapeutic efficacy of anti-PD-1 antibody, anti-PD-L1 antibody and anti-CTLA-4 antibody in cancer therapy.J Transl Med. 2024 Aug 9;22(1):751. doi: 10.1186/s12967-024-05552-6. J Transl Med. 2024. PMID: 39123227 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. . Global Cancer Statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. doi: 10.3322/caac.21660. - PubMed
-
- Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet 2021; 398:535–554. doi: 10.1016/S0140-6736(21)00312-3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

