Cell-based versus corticosteroid injections for knee pain in osteoarthritis: a randomized phase 3 trial
- PMID: 37919438
- PMCID: PMC10719084
- DOI: 10.1038/s41591-023-02632-w
Cell-based versus corticosteroid injections for knee pain in osteoarthritis: a randomized phase 3 trial
Erratum in
-
Author Correction: Cell-based versus corticosteroid injections for knee pain in osteoarthritis: a randomized phase 3 trial.Nat Med. 2024 Mar;30(3):905. doi: 10.1038/s41591-023-02776-9. Nat Med. 2024. PMID: 38135824 Free PMC article. No abstract available.
Abstract
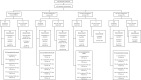
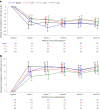
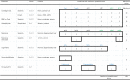
Various types of cellular injection have become a popular and costly treatment option for patients with knee osteoarthritis despite a paucity of literature establishing relative efficacy to each other or corticosteroid injections. Here we aimed to identify the safety and efficacy of cell injections from autologous bone marrow aspirate concentrate, autologous adipose stromal vascular fraction and allogeneic human umbilical cord tissue-derived mesenchymal stromal cells, in comparison to corticosteroid injection (CSI). The study was a phase 2/3, four-arm parallel, multicenter, single-blind, randomized, controlled clinical trial with 480 patients with a diagnosis of knee osteoarthritis (Kellgren-Lawrence II-IV). Participants were randomized to the three different arms with a 3:1 distribution. Arm 1: autologous bone marrow aspirate concentrate (n = 120), CSI (n = 40); arm 2: umbilical cord tissue-derived mesenchymal stromal cells (n = 120), CSI (n = 40); arm 3: stromal vascular fraction (n = 120), CSI (n = 40). The co-primary endpoints were the visual analog scale pain score and Knee injury and Osteoarthritis Outcome Score pain score at 12 months versus baseline. Analyses of our primary endpoints, with 440 patients, revealed that at 1 year post injection, none of the three orthobiologic injections was superior to another, or to the CSI control. In addition, none of the four groups showed a significant change in magnetic resonance imaging osteoarthritis score compared to baseline. No procedure-related serious adverse events were reported during the study period. In summary, this study shows that at 1 year post injection, there was no superior orthobiologic as compared to CSI for knee osteoarthritis. ClinicalTrials.gov Identifier: NCT03818737.
© 2023. The Author(s).
Conflict of interest statement
Several of our authors work for Sanford Health. Sanford Health has a financial interest in InGeneron, Inc., the SVF company used in this study. None of the individual physician has a financial conflict or relationship with InGeneron, Inc. K.M. is a consultant for Lipogems, which is a micronized fat company, not used in this current study. P.C., G.G., L.K., J.K., K.R. and C.Y. all receive separate grant/salary support from the Marcus Foundation, who funded this study.
Figures
Similar articles
-
Clinical Efficacy and Safety of the Intra-articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial.Am J Sports Med. 2023 Jul;51(9):2243-2253. doi: 10.1177/03635465231179223. Epub 2023 Jun 21. Am J Sports Med. 2023. PMID: 37345256 Clinical Trial.
-
Clinical Outcomes of Knee Osteoarthritis Treated With an Autologous Protein Solution Injection: A 1-Year Pilot Double-Blinded Randomized Controlled Trial.Am J Sports Med. 2018 Jan;46(1):171-180. doi: 10.1177/0363546517732734. Epub 2017 Oct 10. Am J Sports Med. 2018. PMID: 29016185 Clinical Trial.
-
Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial.Int Orthop. 2019 May;43(5):1123-1134. doi: 10.1007/s00264-018-4099-0. Epub 2018 Aug 14. Int Orthop. 2019. PMID: 30109404 Clinical Trial.
-
Effect of intra-knee injection of autologous adipose stem cells or mesenchymal vascular components on short-term outcomes in patients with knee osteoarthritis: an updated meta-analysis of randomized controlled trials.Arthritis Res Ther. 2023 Aug 10;25(1):147. doi: 10.1186/s13075-023-03134-3. Arthritis Res Ther. 2023. PMID: 37563715 Free PMC article. Review.
-
The efficacy of intra-articular injections in the treatment of knee osteoarthritis: A network meta-analysis of randomized controlled trials.Knee. 2021 Oct;32:173-182. doi: 10.1016/j.knee.2021.08.008. Epub 2021 Sep 6. Knee. 2021. PMID: 34500430 Review.
Cited by
-
Is cell therapy no better than steroid injection?Nat Rev Rheumatol. 2024 Apr;20(4):199-200. doi: 10.1038/s41584-024-01082-z. Nat Rev Rheumatol. 2024. PMID: 38273002 No abstract available.
-
A programmable arthritis-specific receptor for guided articular cartilage regenerative medicine.bioRxiv [Preprint]. 2024 Feb 15:2024.01.31.578281. doi: 10.1101/2024.01.31.578281. bioRxiv. 2024. Update in: Osteoarthritis Cartilage. 2024 Dec 18:S1063-4584(24)01501-2. doi: 10.1016/j.joca.2024.12.002 PMID: 38352576 Free PMC article. Updated. Preprint.
-
Allogenic bone marrow-derived mesenchymal stromal cell-based therapy for patients with chronic low back pain: a prospective, multicentre, randomised placebo controlled trial (RESPINE study).Ann Rheum Dis. 2024 Oct 21;83(11):1572-1583. doi: 10.1136/ard-2024-225771. Ann Rheum Dis. 2024. PMID: 39393844 Free PMC article. Clinical Trial.
-
Injection therapy in knee osteoarthritis: cortisol, hyaluronic acid, PRP, or BMAC (mesenchymal stem cell therapy)?Front Med (Lausanne). 2024 Sep 27;11:1463997. doi: 10.3389/fmed.2024.1463997. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39399118 Free PMC article. No abstract available.
-
The Exosomes of Stem Cells from Human Exfoliated Deciduous Teeth Suppress Inflammation in Osteoarthritis.Int J Mol Sci. 2024 Aug 6;25(16):8560. doi: 10.3390/ijms25168560. Int J Mol Sci. 2024. PMID: 39201248 Free PMC article.
References
-
- Buckwalter, J. A., Saltzman, C., & Brown, T. The impact of osteoarthritis: implications for research. Clin. Orthop. Relat. Res. 10.1097/01.blo.0000143938.30681.9d (2004). - PubMed

