Ginsenoside Rb1 enhanced immunity and altered the gut microflora in mice immunized by H1N1 influenza vaccine
- PMID: 37868069
- PMCID: PMC10588687
- DOI: 10.7717/peerj.16226
Ginsenoside Rb1 enhanced immunity and altered the gut microflora in mice immunized by H1N1 influenza vaccine
Abstract
Background: Influenza is an acute infectious respiratory disease caused by the influenza virus that seriously damages human health, and the essential way to prevent influenza is the influenza vaccine. Vaccines without adjuvants produce insufficient specific antibodies and therefore require adjuvants to boost antibody titers. Microbes and hosts are a community that needs to "promote bacteria," which could provide new value for the immune effect.
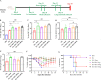
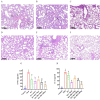
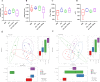
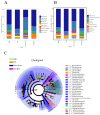
Methods: (1) The H1N1 influenza vaccine, in combination with Ginsenoside Rb1, was co-injected into mice intraperitoneally (I.P.). Then, immunoglobulin G and antibody subtype levels were tested by enzyme-linked immunosorbent assay (ELISA). Moreover, mice were infected with a lethal dose of the H1N1 influenza virus (A/Michigan/45/2015), and survival status was recorded for 14 days. Lung tissues were stained by hematoxylin and eosin (H&E), and ELISA detected inflammatory factor expression levels. (2) Mice were immunized with Ginsenoside Rb1 combined with quadrivalent influenza inactivated vaccine(IIV4), and then IgG levels were measured by ELISA. (3) Fresh stool was collected for fecal 16S rDNA analysis.
Results: Ginsenoside Rb1 boosted IgG and antibody subtypes in the H1N1 influenza vaccine, improved survival of mice after virus challenge, attenuated lung histopathological damage, and reduced inflammatory cytokines expression in IL-6 and TNF-α. The results of 16S rDNA showed that Rb1 decreased species diversity but increased species richness compared to the PBS group and increased the abundance of Akkermansiaceae and Murbaculaceae at the Family and Genus levels compared with the HA+Alum group.
Conclusion: Ginsenoside Rb1 has a boosting effect on the immune efficacy of the H1N1 influenza vaccine and is promising as a novel adjuvant to regulate the microecological balance and achieve an anti-infective effect.
Keywords: 16S rRNA; Adjuvant; Ginsenoside Rb1; Gut microbiota; Polysaccharide.
©2023 Wan et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

Similar articles
-
Astragalus Polysaccharide improves immunogenicity of influenza vaccine as well as modulate gut microbiota in BALB/c mice.Microb Pathog. 2024 Oct;195:106893. doi: 10.1016/j.micpath.2024.106893. Epub 2024 Aug 27. Microb Pathog. 2024. PMID: 39197333
-
Characterization of cross protection of Swine-Origin Influenza Virus (S-OIV) H1N1 and reassortant H5N1 influenza vaccine in BALB/c mice given a single-dose vaccination.J Biomed Sci. 2013 Mar 21;20(1):19. doi: 10.1186/1423-0127-20-19. J Biomed Sci. 2013. PMID: 23517052 Free PMC article.
-
Mannan adjuvants intranasally administered inactivated influenza virus in mice rendering low doses inductive of strong serum IgG and IgA in the lung.BMC Infect Dis. 2015 Feb 26;15:101. doi: 10.1186/s12879-015-0838-7. BMC Infect Dis. 2015. PMID: 25887952 Free PMC article.
-
Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration.Vaccine. 2012 Jul 6;30(32):4884-91. doi: 10.1016/j.vaccine.2012.04.032. Epub 2012 Apr 23. Vaccine. 2012. PMID: 22537989
-
Cross-protective immunity against influenza A/H1N1 virus challenge in mice immunized with recombinant vaccine expressing HA gene of influenza A/H5N1 virus.Virol J. 2013 Sep 22;10:291. doi: 10.1186/1743-422X-10-291. Virol J. 2013. PMID: 24053449 Free PMC article.
Cited by
-
Gut aging: A wane from the normal to repercussion and gerotherapeutic strategies.Heliyon. 2024 Sep 12;10(19):e37883. doi: 10.1016/j.heliyon.2024.e37883. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39381110 Free PMC article. Review.
-
Ginsenoside Rb1 reduced ischemic stroke-induced apoptosis through endoplasmic reticulum stress-associated IRE1/TRAF2/JNK pathway.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jan;398(1):747-764. doi: 10.1007/s00210-024-03292-4. Epub 2024 Jul 25. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39052059
References
-
- Akatsu H, Nagafuchi S, Kurihara R, Okuda K, Kanesaka T, Ogawa N, Kanematsu T, Takasugi S, Yamaji T, Takami M, Yamamoto T, Ohara H, Maruyama M. Enhanced vaccination effect against influenza by prebiotics in elderly patients receiving enteral nutrition. Geriatrics & Gerontology International. 2016;16:205–213. doi: 10.1111/ggi.12454. - DOI - PubMed
-
- Bai Y, Bao X, Mu Q, Fang X, Zhu R, Liu C, Mo F, Zhang D, Jiang G, Li P, Gao S, Zhao D. Ginsenoside Rb1, salvianolic acid B and their combination modulate gut microbiota and improve glucolipid metabolism in high-fat diet induced obese mice. PeerJ. 2021;9:e10598. doi: 10.7717/peerj.10598. - DOI - PMC - PubMed
-
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. - DOI - PMC - PubMed
-
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

