RILP inhibits tumor progression in osteosarcoma via Grb10-mediated inhibition of the PI3K/AKT/mTOR pathway
- PMID: 37789274
- PMCID: PMC10548720
- DOI: 10.1186/s10020-023-00722-6
RILP inhibits tumor progression in osteosarcoma via Grb10-mediated inhibition of the PI3K/AKT/mTOR pathway
Abstract
Background: Rab-interacting lysosomal protein (RILP) contains an alpha-helical coil with an unexplored biological function in osteosarcoma. This study investigated the expression of RILP in osteosarcoma cells and tissues to determine the effect of RILP on the biological behaviors of osteosarcoma cells and the underlying mechanism.
Methods: Tumor Immune Estimation Resource (TIMER) database, The Cancer Genome Atlas (TCGA) database and Gene Expression Omnibus (GEO) database were used for bioinformatic analysis. Co-immunoprecipitation experiment was used to determine whether the two proteins were interacting. In functional tests, cell counting kit-8 (CCK-8) assay, colony formation assay, wound healing assay, transwell invasion assay, Immunofluorescence (IF) assay and immunohistochemical (IHC) assay were performed.
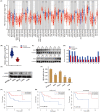
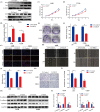
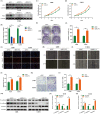
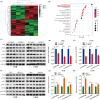
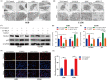
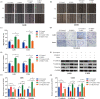
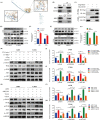
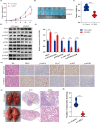
Results: Overexpression of RILP significantly inhibited proliferation and impaired metastasis ability of osteosarcoma cells, while silencing of RILP showed the opposite trend. RNA-seq data analysis was applied in 143B cells and pathway enrichment analysis revealed that differentially expressed genes were mainly enriched in the PI3K/AKT pathway. We further verified that overexpression of RILP restrained the PI3K/AKT/mTOR signaling pathway and induced autophagy in osteosarcoma cells, while the opposite trend was observed when PI3K pathway activator 740Y-P was used. 3-Methyladenine (3-MA), a selective autophagy inhibitor, partially attenuated the inhibitory effect of RILP on the migration and invasion ability of osteosarcoma cells, suggesting the involvement of autophagy in epithelial-mesenchymal transition regulation in osteosarcoma cells. Growth factor receptor binding protein-10 (Grb10), an adaptor protein, was confirmed as a potential target of RILP to restrain the PI3K/AKT signaling pathway. We subcutaneously injected stably overexpressing 143B osteosarcoma cells into nude mice and observed that overexpression of RILP inhibited tumor growth by inhibiting the PI3K/AKT/mTOR pathway.
Conclusion: Our study revealed that the expression of RILP was associated with favorable prognosis of osteosarcoma and RILP inhibits proliferation, migration, and invasion and promotes autophagy in osteosarcoma cells via Grb10-mediated inhibition of the PI3K/AKT/mTOR signaling pathway. In the future, targeting RILP may be a potential strategy for osteosarcoma treatment.
Keywords: Autophagy; Epithelial–mesenchymal transition; Osteosarcoma; RILP.
© 2023. The Feinstein Institute for Medical Research.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
The role of programmed cell death in osteosarcoma: From pathogenesis to therapy.Cancer Med. 2024 May;13(10):e7303. doi: 10.1002/cam4.7303. Cancer Med. 2024. PMID: 38800967 Free PMC article. Review.
-
SLC38A5 suppresses ferroptosis through glutamine-mediated activation of the PI3K/AKT/mTOR signaling in osteosarcoma.J Transl Med. 2024 Nov 7;22(1):1004. doi: 10.1186/s12967-024-05803-6. J Transl Med. 2024. PMID: 39511570 Free PMC article.
-
AIM2 inhibits the proliferation, invasion and migration, and promotes the apoptosis of osteosarcoma cells by inactivating the PI3K/AKT/mTOR signaling pathway.Mol Med Rep. 2022 Feb;25(2):53. doi: 10.3892/mmr.2021.12569. Epub 2021 Dec 16. Mol Med Rep. 2022. PMID: 34913077 Free PMC article.
-
MicroRNA‑22 mediates the cisplatin resistance of osteosarcoma cells by inhibiting autophagy via the PI3K/Akt/mTOR pathway.Oncol Rep. 2020 Apr;43(4):1169-1186. doi: 10.3892/or.2020.7492. Epub 2020 Feb 7. Oncol Rep. 2020. PMID: 32323781 Free PMC article.
-
Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma.Open Life Sci. 2024 Aug 6;19(1):20220936. doi: 10.1515/biol-2022-0936. eCollection 2024. Open Life Sci. 2024. PMID: 39119480 Free PMC article. Review.
Cited by
-
Synergistic effects of bloom helicase (BLM) inhibitor AO/854 with cisplatin in prostate cancer.Sci Rep. 2024 Oct 23;14(1):24962. doi: 10.1038/s41598-024-75938-5. Sci Rep. 2024. PMID: 39438537 Free PMC article.
-
Lysosomes in Cancer-At the Crossroad of Good and Evil.Cells. 2024 Mar 5;13(5):459. doi: 10.3390/cells13050459. Cells. 2024. PMID: 38474423 Free PMC article. Review.
-
RILP Induces Cholesterol Accumulation in Lysosomes by Inhibiting Endoplasmic Reticulum-Endolysosome Interactions.Cells. 2024 Aug 6;13(16):1313. doi: 10.3390/cells13161313. Cells. 2024. PMID: 39195203 Free PMC article.
-
The role of programmed cell death in osteosarcoma: From pathogenesis to therapy.Cancer Med. 2024 May;13(10):e7303. doi: 10.1002/cam4.7303. Cancer Med. 2024. PMID: 38800967 Free PMC article. Review.
-
Apoptosis and Inflammation Involved with Fluoride-Induced Bone Injuries.Nutrients. 2024 Jul 31;16(15):2500. doi: 10.3390/nu16152500. Nutrients. 2024. PMID: 39125380 Free PMC article.
References
-
- Corti F, Nichetti F, Raimondi A, Niger M, Prinzi N, Torchio M, Tamborini E, Perrone F, Pruneri G, Di Bartolomeo M, et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: a review of current evidences and future perspectives. Cancer Treat Rev. 2019;72:45–55. doi: 10.1016/j.ctrv.2018.11.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

