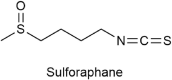
Sulforaphane and bladder cancer: a potential novel antitumor compound
- PMID: 37781700
- PMCID: PMC10540234
- DOI: 10.3389/fphar.2023.1254236
Sulforaphane and bladder cancer: a potential novel antitumor compound
Abstract
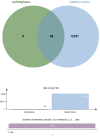
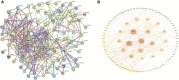
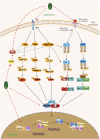
Bladder cancer (BC) is a common form of urinary tract tumor, and its incidence is increasing annually. Unfortunately, an increasing number of newly diagnosed BC patients are found to have advanced or metastatic BC. Although current treatment options for BC are diverse and standardized, it is still challenging to achieve ideal curative results. However, Sulforaphane, an isothiocyanate present in cruciferous plants, has emerged as a promising anticancer agent that has shown significant efficacy against various cancers, including bladder cancer. Recent studies have demonstrated that Sulforaphane not only induces apoptosis and cell cycle arrest in BC cells, but also inhibits the growth, invasion, and metastasis of BC cells. Additionally, it can inhibit BC gluconeogenesis and demonstrate definite effects when combined with chemotherapeutic drugs/carcinogens. Sulforaphane has also been found to exert anticancer activity and inhibit bladder cancer stem cells by mediating multiple pathways in BC, including phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK), nuclear factor kappa-B (NF-κB), nuclear factor (erythroid-derived 2)-like 2 (Nrf2), zonula occludens-1 (ZO-1)/beta-catenin (β-Catenin), miR-124/cytokines interleukin-6 receptor (IL-6R)/transcription 3 (STAT3). This article provides a comprehensive review of the current evidence and molecular mechanisms of Sulforaphane against BC. Furthermore, we explore the effects of Sulforaphane on potential risk factors for BC, such as bladder outlet obstruction, and investigate the possible targets of Sulforaphane against BC using network pharmacological analysis. This review is expected to provide a new theoretical basis for future research and the development of new drugs to treat BC.
Keywords: MAPK; NF-κB; bladder cancer; bladder outlet obstruction; sulforaphane.
Copyright © 2023 Zuo, Chen, Liao, He, Xu, Tang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Sulforaphane induces apoptosis in T24 human urinary bladder cancer cells through a reactive oxygen species-mediated mitochondrial pathway: the involvement of endoplasmic reticulum stress and the Nrf2 signaling pathway.Int J Oncol. 2014 Oct;45(4):1497-506. doi: 10.3892/ijo.2014.2536. Epub 2014 Jul 4. Int J Oncol. 2014. PMID: 24993616
-
Antitumor activity of sulforaphane in mice model of skin cancer via blocking sulfatase-2.Exp Dermatol. 2019 Jan;28(1):28-34. doi: 10.1111/exd.13802. Epub 2018 Dec 11. Exp Dermatol. 2019. PMID: 30315662
-
Sulforaphane down-regulates COX-2 expression by activating p38 and inhibiting NF-kappaB-DNA-binding activity in human bladder T24 cells.Int J Oncol. 2009 Apr;34(4):1129-34. doi: 10.3892/ijo_00000240. Int J Oncol. 2009. PMID: 19287971
-
Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress.J Adv Res. 2021 Jul 6;34:43-63. doi: 10.1016/j.jare.2021.06.023. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024180 Free PMC article. Review.
-
Sulforaphane for the chemoprevention of bladder cancer: molecular mechanism targeted approach.Oncotarget. 2017 May 23;8(21):35412-35424. doi: 10.18632/oncotarget.16015. Oncotarget. 2017. PMID: 28423681 Free PMC article. Review.
References
-
- Abbaoui B., Riedl K. M., Ralston R. A., Thomas-Ahner J. M., Schwartz S. J., Clinton S. K., et al. (2012). Inhibition of bladder cancer by broccoli isothiocyanates sulforaphane and erucin: characterization, metabolism, and interconversion. Mol. Nutr. Food. Res. 56 (11), 1675–1687. 10.1002/mnfr.201200276 - DOI - PMC - PubMed
-
- Abbaoui B., Telu K. H., Lucas C. R., Thomas-Ahner J. M., Schwartz S. J., Clinton S. K., et al. (2017). The impact of cruciferous vegetable isothiocyanates on histone acetylation and histone phosphorylation in bladder cancer. J. Proteomics. 156, 94–103. 10.1016/j.jprot.2017.01.013 - DOI - PMC - PubMed
-
- Abdull Razis A. F., Bagatta M., De Nicola G. R., Iori R., Ioannides C. (2011b). Induction of epoxide hydrolase and glucuronosyl transferase by isothiocyanates and intact glucosinolates in precision-cut rat liver slices: importance of side-chain substituent and chirality. Arch. Toxicol. 85 (8), 919–927. 10.1007/s00204-010-0629-2 - DOI - PubMed
-
- Akbari E., Namazian M. (2020). Sulforaphane: A natural product against reactive oxygen species. Comput. Theor. Chem. 1183, 112850. 10.1016/j.comptc.2020.112850 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

