Repositioning of clinically approved drug Bazi Bushen capsule for treatment of Aizheimer's disease using network pharmacology approach and in vitro experimental validation
- PMID: 37449101
- PMCID: PMC10336525
- DOI: 10.1016/j.heliyon.2023.e17603
Repositioning of clinically approved drug Bazi Bushen capsule for treatment of Aizheimer's disease using network pharmacology approach and in vitro experimental validation
Abstract
Aims: To explore the new indications and key mechanism of Bazi Bushen capsule (BZBS) by network pharmacology and in vitro experiment.
Methods: The ingredients library of BZBS was constructed by retrieving multiple TCM databases. The potential target profiles of the components were predicted by target prediction algorithms based on different principles, and validated by using known activity data. The target spectrum of BZBS with high reliability was screened by considering the source of the targets and the node degree in compound-target (C-T) network. Subsequently, new indications for BZBS were predicted by disease ontology (DO) enrichment analysis and initially validated by GO and KEGG pathway enrichment analysis. Furthermore, the target sets of BZBS acting on AD signaling pathway were identified by intersection analysis. Based on STRING database, the PPI network of target was constructed and their node degree was calculated. Two Alzheimer's disease (AD) cell models, BV-2 and SH-SY5Y, were used to preliminarily verify the anti-AD efficacy and mechanism of BZBS in vitro.
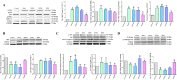
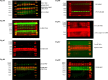
Results: In total, 1499 non-repeated ingredients were obtained from 16 herbs in BZBS formula, and 1320 BZBS targets with high confidence were predicted. Disease enrichment results strongly suggested that BZBS formula has the potential to be used in the treatment of AD. GO and KEGG enrichment results provide a preliminary verification of this point. Among them, 113 functional targets of BZBS belong to AD pathway. A PPI network containing 113 functional targets and 1051 edges for the treatment of AD was constructed. In vitro experiments showed that BZBS could significantly reduce the release of TNF-α and IL-6 and the expression of COX-2 and PSEN1 in Aβ25-35-induced BV-2 cells, which may be related to the regulation of ERK1/2/NF-κB signaling pathway. BZBS reduced the apoptosis rate of Aβ25-35 induced SH-SY5Y cells, significantly increased mitochondrial membrane potential, reduced the expression of Caspase3 active fragment and PSEN1, and increased the expression of IDE. This may be related to the regulation of GSK-3β/β-catenin signaling pathway.
Conclusions: BZBS formula has a potential use in the treatment of AD, which is achieved through regulation of ERK1/2, NF-κB signaling pathways, and GSK-3β/β-catenin signaling pathway. Furthermore, the network pharmacology technology is a feasible drug repurposing strategy to reposition new clinical use of approved TCM and explore the mechanism of action. The study lays a foundation for the subsequent in-depth study of BZBS in the treatment of AD and provides a basis for its application in the clinical treatment of AD.
Keywords: Aizheimer's disease; Bazi Bushen capsule; Drug repositioning; Mechanism of action; Network pharmacology; Network targets.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


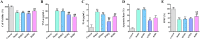
Similar articles
-
Bazi Bushen capsule attenuates cardiac systolic injury via SIRT3/SOD2 pathway in high-fat diet-fed ovariectomized mice.Heliyon. 2024 May 31;10(11):e32159. doi: 10.1016/j.heliyon.2024.e32159. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38912487 Free PMC article.
-
Mechanism of Bazi Bushen capsule in delaying the senescence of mesenchymal stem cells based on network pharmacology and experimental validation.Heliyon. 2024 Mar 9;10(6):e27646. doi: 10.1016/j.heliyon.2024.e27646. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38509951 Free PMC article.
-
[Mechanism of Berberis atrocarpa anthocyanin against Alzheimer's disease based on network pharmacology and experimental verification].Zhongguo Zhong Yao Za Zhi. 2023 Feb;48(3):778-788. doi: 10.19540/j.cnki.cjcmm.20220905.706. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 36872242 Chinese.
-
Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis.Comput Biol Med. 2022 May;144:105389. doi: 10.1016/j.compbiomed.2022.105389. Epub 2022 Mar 9. Comput Biol Med. 2022. PMID: 35303581 Review.
-
Exploration of the mechanism of Zisheng Shenqi decoction against gout arthritis using network pharmacology.Comput Biol Chem. 2021 Feb;90:107358. doi: 10.1016/j.compbiolchem.2020.107358. Epub 2020 Aug 8. Comput Biol Chem. 2021. PMID: 33243703 Review.
Cited by
-
Bazi Bushen capsule attenuates cardiac systolic injury via SIRT3/SOD2 pathway in high-fat diet-fed ovariectomized mice.Heliyon. 2024 May 31;10(11):e32159. doi: 10.1016/j.heliyon.2024.e32159. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38912487 Free PMC article.
-
Gut aging: A wane from the normal to repercussion and gerotherapeutic strategies.Heliyon. 2024 Sep 12;10(19):e37883. doi: 10.1016/j.heliyon.2024.e37883. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39381110 Free PMC article. Review.
-
Mechanism of Bazi Bushen capsule in delaying the senescence of mesenchymal stem cells based on network pharmacology and experimental validation.Heliyon. 2024 Mar 9;10(6):e27646. doi: 10.1016/j.heliyon.2024.e27646. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38509951 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

