A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids
- PMID: 37446644
- PMCID: PMC10343696
- DOI: 10.3390/molecules28134982
A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids
Abstract
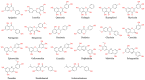
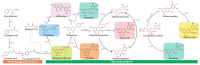
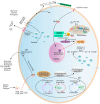
Flavonoids represent the main class of plant secondary metabolites and occur in the tissues and organs of various plant species. In plants, flavonoids are involved in many biological processes and in response to various environmental stresses. The consumption of flavonoids has been known to reduce the risk of many chronic diseases due to their antioxidant and free radical scavenging properties. In the present review, we summarize the classification, distribution, biosynthesis pathways, and regulatory mechanisms of flavonoids. Moreover, we investigated their biological activities and discuss their applications in food processing and cosmetics, as well as their pharmaceutical and medical uses. Current trends in flavonoid research are also briefly described, including the mining of new functional genes and metabolites through omics research and the engineering of flavonoids using nanotechnology. This review provides a reference for basic and applied research on flavonoid compounds.
Keywords: application; biological activity; biosynthesis pathway; classification; flavonoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Flavonoid Biosynthesis Network in Plants.Int J Mol Sci. 2021 Nov 26;22(23):12824. doi: 10.3390/ijms222312824. Int J Mol Sci. 2021. PMID: 34884627 Free PMC article. Review.
-
Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity.Food Chem. 2022 Jul 30;383:132531. doi: 10.1016/j.foodchem.2022.132531. Epub 2022 Feb 23. Food Chem. 2022. PMID: 35413752 Review.
-
Accumulation characteristics of plant flavonoids and effects of cultivation measures on their biosynthesis: A review.Plant Physiol Biochem. 2024 Oct;215:108960. doi: 10.1016/j.plaphy.2024.108960. Epub 2024 Jul 23. Plant Physiol Biochem. 2024. PMID: 39079230 Review.
-
The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses.Molecules. 2023 Apr 20;28(8):3599. doi: 10.3390/molecules28083599. Molecules. 2023. PMID: 37110833 Free PMC article. Review.
-
Biocatalytic Synthesis of Flavonoid Esters by Lipases and Their Biological Benefits.Planta Med. 2017 Jan;83(1-02):7-22. doi: 10.1055/s-0042-118883. Epub 2016 Dec 5. Planta Med. 2017. PMID: 27919108 Review.
Cited by
-
Dietary Polyphenols, Food Processing and Gut Microbiome: Recent Findings on Bioavailability, Bioactivity, and Gut Microbiome Interplay.Antioxidants (Basel). 2024 Oct 10;13(10):1220. doi: 10.3390/antiox13101220. Antioxidants (Basel). 2024. PMID: 39456473 Free PMC article. Review.
-
Chemical Constituents and Biological Activities of Bruguiera Genus and Its Endophytes: A Review.Mar Drugs. 2024 Mar 29;22(4):158. doi: 10.3390/md22040158. Mar Drugs. 2024. PMID: 38667775 Free PMC article. Review.
-
Natural Compounds in Non-Melanoma Skin Cancer: Prevention and Treatment.Molecules. 2024 Feb 4;29(3):728. doi: 10.3390/molecules29030728. Molecules. 2024. PMID: 38338469 Free PMC article. Review.
-
Puerarin-A Promising Flavonoid: Biosynthesis, Extraction Methods, Analytical Techniques, and Biological Effects.Int J Mol Sci. 2024 May 10;25(10):5222. doi: 10.3390/ijms25105222. Int J Mol Sci. 2024. PMID: 38791264 Free PMC article. Review.
-
Effects of Phenolic Acids Produced from Food-Derived Flavonoids and Amino Acids by the Gut Microbiota on Health and Disease.Molecules. 2024 Oct 29;29(21):5102. doi: 10.3390/molecules29215102. Molecules. 2024. PMID: 39519743 Free PMC article. Review.
References
-
- Santos E.L., Maia B., Ferriani A.P., Teixeira S.D. Flavonoids: From Biosynthesis to Human Health. Volume 13. IntechOpen; London, UK: 2017. Flavonoids: Classification, biosynthesis and chemical ecology; pp. 78–94. - DOI
-
- Karak P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019;10:1567–1574. doi: 10.13040/IJPSR.0975-8232.10(4).1567-74. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

