Dose-dependent effect of GFI1 expression in the reconstitution and the differentiation capacity of HSCs
- PMID: 37091981
- PMCID: PMC10113925
- DOI: 10.3389/fcell.2023.866847
Dose-dependent effect of GFI1 expression in the reconstitution and the differentiation capacity of HSCs
Abstract
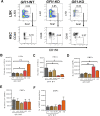
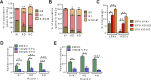
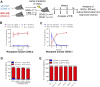
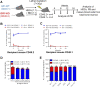
GFI1 is a transcriptional repressor and plays a pivotal role in regulating the differentiation of hematopoietic stem cells (HSCs) towards myeloid and lymphoid cells. Serial transplantation of Gfi1 deficient HSCs repopulated whole hematopoietic system but in a competitive setting involving wild-type HSCs, they lose this ability. The underlying mechanisms to this end are poorly understood. To better understand this, we used different mouse strains that express either loss of both Gfi1 alleles (Gfi1-KO), with reduced expression of GFI1 (GFI1-KD) or wild-type Gfi1/GFI1 (Gfi1-/GFI1-WT; corresponding to the mouse and human alleles). We observed that loss of Gfi1 or reduced expression of GFI1 led to a two to four fold lower number of HSCs (defined as Lin-Sca1+c-Kit+CD150+CD48-) compared to GFI1-WT mice. To study the functional influence of different levels of GFI1 expression on HSCs function, HSCs from Gfi1-WT (expressing CD45.1 + surface antigens) and HSCs from GFI1-KD or -KO (expressing CD45.2 + surface antigens) mice were sorted and co-transplanted into lethally irradiated host mice. Every 4 weeks, CD45.1+ and CD45.2 + on different lineage mature cells were analyzed by flow cytometry. At least 16 weeks later, mice were sacrificed, and the percentage of HSCs and progenitors including GMPs, CMPs and MEPs in the total bone marrow cells was calculated as well as their CD45.1 and CD45.2 expression. In the case of co-transplantation of GFI1-KD with Gfi1-WT HSCs, the majority of HSCs (81% ± 6%) as well as the majority of mature cells (88% ± 10%) originated from CD45.2 + GFI1-KD HSCs. In the case of co-transplantation of Gfi1-KO HSCs with Gfi1-WT HSCs, the majority of HSCs originated from CD45.2+ and therefore from Gfi1-KO (61% ± 20%); however, only a small fraction of progenitors and mature cells originated from Gfi1-KO HSCs (<1%). We therefore in summary propose that GFI1 has a dose-dependent role in the self-renewal and differentiation of HSCs.
Keywords: Gfi1; HSC; differentiation; dose-dependent; engraftment.
Copyright © 2023 Xie, Patnana, Frank, Schütte, Al-Matary, Künstner, Busch, Ahmed, Liu, Engel, Dührsen, Rosenbauer, Von Bubnoff, Lenz and Khandanpour.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells.EMBO J. 2004 Oct 13;23(20):4116-25. doi: 10.1038/sj.emboj.7600419. Epub 2004 Sep 23. EMBO J. 2004. PMID: 15385956 Free PMC article.
-
Growth factor independence 1 protects hematopoietic stem cells against apoptosis but also prevents the development of a myeloproliferative-like disease.Stem Cells. 2011 Feb;29(2):376-85. doi: 10.1002/stem.575. Stem Cells. 2011. PMID: 21732494
-
Multifaceted Actions of GFI1 and GFI1B in Hematopoietic Stem Cell Self-Renewal and Lineage Commitment.Front Genet. 2020 Oct 26;11:591099. doi: 10.3389/fgene.2020.591099. eCollection 2020. Front Genet. 2020. PMID: 33193732 Free PMC article. Review.
-
Loss of IKKβ but Not NF-κB p65 Skews Differentiation towards Myeloid over Erythroid Commitment and Increases Myeloid Progenitor Self-Renewal and Functional Long-Term Hematopoietic Stem Cells.PLoS One. 2015 Jun 23;10(6):e0130441. doi: 10.1371/journal.pone.0130441. eCollection 2015. PLoS One. 2015. PMID: 26102347 Free PMC article.
-
Dormant and self-renewing hematopoietic stem cells and their niches.Ann N Y Acad Sci. 2007 Jun;1106:64-75. doi: 10.1196/annals.1392.021. Epub 2007 Apr 18. Ann N Y Acad Sci. 2007. PMID: 17442778 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

