Combination Treatment with Sipuleucel-T and Abiraterone Acetate or Enzalutamide for Metastatic Castration-Resistant Prostate Cancer: STAMP and STRIDE Trials
- PMID: 37058234
- PMCID: PMC10320463
- DOI: 10.1158/1078-0432.CCR-22-3832
Combination Treatment with Sipuleucel-T and Abiraterone Acetate or Enzalutamide for Metastatic Castration-Resistant Prostate Cancer: STAMP and STRIDE Trials
Abstract
Purpose: We present long-term outcomes from 2 randomized studies [STAMP (with abiraterone, NCT01487863) and STRIDE (with enzalutamide, NCT01981122)] that were performed to study the impact of sequential or concurrent administration of androgen receptor-targeting agents (ARTAs) on sipuleucel-T immune response and overall survival (OS) in metastatic castration-resistant prostate cancer (mCRPC).
Patients and methods: Sipuleucel-T was administered per current prescribing information. Results from STRIDE are presented together with updated STAMP results. Survival status of patients was updated using demographic information to query the National Death Index (NDI). Kaplan-Meier methodology was used to analyze survival.
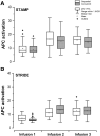
Results: Updated data reduced patient censoring in each study compared with the original analyses; the 95% confidence intervals (CIs) for OS are now estimable. Updated median OS (95% CI) is 33.3 (24.1-40.7) months for STAMP and 32.5 (26.0-45.1) months for STRIDE. There was no notable impact on median OS [HR, 0.727 (0.458-1.155); P = 0.177, reference = STRIDE]. OS with sequential administration was similar to concurrent administration [NDI update: HR, 0.963 (0.639-1.453); P = 0.845, reference = concurrent arm]. Sipuleucel-T potency, measured as antigen-presenting cell (APC) activation, was higher in subsequent infusions compared with the first infusion. Humoral responses (IgG + IgM antibody titers) to PA2024 and prostatic acid phosphatase were significantly elevated versus baseline. No new safety signals were observed.
Conclusions: Median OS was consistent regardless of whether the agents were administered sequentially or concurrently, including after NDI update. Results suggest that sipuleucel-T induces an immunologic prime-boost effect after initial sipuleucel-T exposure, even when combined with ARTAs.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures
![Figure 2. Kaplan–Meier estimates of OS in patients who received an ARTA along with sipuleucel-T, either concurrently or sequentially. Plots of Kaplan–Meier estimates of OS by treatment arm [concurrent (— red solid line) or sequential (- - - blue dashed line)] for STAMP (NCT01487863; abiraterone acetate + prednisone and sipuleucel-T; A) and STRIDE [NCT01981122; enzalutamide and sipuleucel-T (concurrent — red solid line) or sequential (- - - blue dashed line); B]. Plot of Kaplan–Meier estimates of OS by agent as presented by study (C), collapsed across treatment arms within each study: STAMP (— red solid line) and STRIDE (- - - blue dashed line). D, Plot of Kaplan–Meier estimates of OS by treatment paradigm: concurrent (— red solid line) and sequential (- - - blue dashed line). Numbers remaining at risk are presented. Abbreviations: Sx, number of subjects included; Ev, number of events (death); Ce, censored as was lost to follow-up.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/fb86/10320463/02217e1882ac/2426fig2.gif)
Similar articles
-
Real-World Effectiveness of Sipuleucel-T on Overall Survival in Men with Advanced Prostate Cancer Treated with Androgen Receptor-Targeting Agents.Adv Ther. 2022 Jun;39(6):2515-2532. doi: 10.1007/s12325-022-02085-6. Epub 2022 Mar 30. Adv Ther. 2022. PMID: 35352309 Free PMC article.
-
A Randomized Phase II Trial of Sipuleucel-T with Concurrent versus Sequential Abiraterone Acetate plus Prednisone in Metastatic Castration-Resistant Prostate Cancer.Clin Cancer Res. 2015 Sep 1;21(17):3862-9. doi: 10.1158/1078-0432.CCR-15-0079. Epub 2015 Apr 29. Clin Cancer Res. 2015. PMID: 25925891 Clinical Trial.
-
A Retrospective Observational Analysis of Overall Survival with Sipuleucel-T in Medicare Beneficiaries Treated for Advanced Prostate Cancer.Adv Ther. 2020 Dec;37(12):4910-4929. doi: 10.1007/s12325-020-01509-5. Epub 2020 Oct 7. Adv Ther. 2020. PMID: 33029725 Free PMC article.
-
Systematic Review and Network Meta-Analysis of Treatments for Chemotherapy-Naive Patients with Asymptomatic/Mildly Symptomatic Metastatic Castration-Resistant Prostate Cancer.Value Health. 2018 Oct;21(10):1259-1268. doi: 10.1016/j.jval.2018.03.012. Epub 2018 May 4. Value Health. 2018. PMID: 30314628 Review.
-
Treating Patients with Metastatic Castration Resistant Prostate Cancer: A Comprehensive Review of Available Therapies.J Urol. 2015 Dec;194(6):1537-47. doi: 10.1016/j.juro.2015.06.106. Epub 2015 Jul 18. J Urol. 2015. PMID: 26196735 Review.
Cited by
-
Current Trends and Innovative Approaches in Cancer Immunotherapy.AAPS PharmSciTech. 2024 Jul 24;25(6):168. doi: 10.1208/s12249-024-02883-x. AAPS PharmSciTech. 2024. PMID: 39044047 Review.
-
Synergistic Strategies for Castration-Resistant Prostate Cancer: Targeting AR-V7, Exploring Natural Compounds, and Optimizing FDA-Approved Therapies.Cancers (Basel). 2024 Aug 6;16(16):2777. doi: 10.3390/cancers16162777. Cancers (Basel). 2024. PMID: 39199550 Free PMC article. Review.
-
Race-Related Differences in Sipuleucel-T Response among Men with Metastatic Castrate-Resistant Prostate Cancer.Cancer Res Commun. 2024 Jul 1;4(7):1715-1725. doi: 10.1158/2767-9764.CRC-24-0112. Cancer Res Commun. 2024. PMID: 38856749 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

