Trends, characteristics, race, and ethnicity associated with nonadherence to antidepressants among breast cancer survivors with depression
- PMID: 36989452
- PMCID: PMC10387908
- DOI: 10.18553/jmcp.2023.29.4.431
Trends, characteristics, race, and ethnicity associated with nonadherence to antidepressants among breast cancer survivors with depression
Abstract
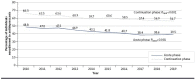
BACKGROUND: Breast cancer is the most diagnosed cancer in the United States, and half of breast cancer survivors experience major depressive disorders (hereafter depression). Healthcare Effectiveness Data and Information Set (HEDIS) quality measures evaluating depression treatment practices recommend uninterrupted antidepressant treatment for 3 months in the acute phase and 3 months in the continuation phase for the general population. However, little is known about the extent of and trends in antidepressant nonadherence among breast cancer survivors with depression, which may impact adherence to breast cancer treatment, potentially leading to breast cancer recurrence and other adverse outcomes. OBJECTIVE: To examine the trends and characteristics associated with antidepressant nonadherence among breast cancer survivors with depression in the United States. METHODS: We conducted cross-sectional analyses of Surveillance, Epidemiology, and End Results linked with Medicare data (2010-2019) for women with breast cancer and depression who newly initiated antidepressant use. Using HEDIS measures of nonadherence (ie, antidepressant prescription coverage ≤84 days of the 114-day acute phase or ≤180 days of the 231-day continuation phase), we calculated the annual crude prevalence of antidepressant nonadherence and examined trends using unadjusted logistic regression. Multivariable logistic regression identified characteristics associated with antidepressant nonadherence. RESULTS: Among 9,452 eligible breast cancer survivors with depression (aged ≥65 years = 84% and White race = 82%), the crude prevalence of antidepressant nonadherence decreased from 2010 to 2019 for both the acute (49% to 40%; Ptrend<0.001) and continuation (67% to 57%; Ptrend<0.001) phases. Factors significantly associated with higher odds of antidepressant nonadherence in both the acute and continuation phases included Black race (odds ratios [ORs] [95% CI] for the acute/continuation phases: 2.0 [1.7-2.4]/2.0 [1.7-2.3]) and Hispanic ethnicity (1.5 [1.1-1.9]/2.2 [1.6-2.9]) compared with White race; receiving the first antidepressant from an oncologist vs a psychiatrist (1.4 [1.1-1.8]/1.6 [1.2-2.0]); and using antidepressants not recommended for older adults by the Beers criteria (2.2 [1.6-2.9]/2.0 [1.4-2.7]). Factors associated with lower odds of antidepressant nonadherence in both phases included receiving lymph node dissection (0.7 [0.5-0.9]/0.7 [0.5-0.9]), receiving endocrine therapy (0.9 [0.8-0.9]/0.8 [0.7-0.9]), having a higher National Cancer Institute comorbid index (0.8 [0.7-0.8]/0.9 [0.8-0.9]), having a follow-up visit with a psychiatrist (0.9 [0.8-0.9]/0.9 [0.8-0.9]), and switching to different antidepressants (0.7 [0.6-0.8]/0.7 [0.7-0.8]). CONCLUSIONS: Despite antidepressant nonadherence prevalence decreasing from 2010 to 2019, over half of breast cancer survivors with depression and Medicare were nonadherent in the continuation phase. Patients with identified nonadherence risk factors may benefit from close monitoring and targeted interventions. DISCLOSURES: Wei-Hsuan Lo-Ciganic reported grants from the National Institute on Drug Abuse (R01DA044985 and R01DA050676), the National Institute on Aging (R21AG060308), the National Institute of Mental Health (R01MH121907), Merck Sharp & Dohme, Bristol Myers Squibb, the Richard King Mellon Foundation at the University of Pittsburgh, the Clinical and Translational Science Institute of the University of Florida, the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation, and the US Department of Veterans Affairs outside the submitted work; in addition, Wei-Hsuan Lo-Ciganic has a patent pending for U1195.70174US00. Haesuk Park reported grants from Bristol Myers Squibb/Pfizer Alliance American Thrombosis Investigator Initiated Research Program (ARISTA-USA) outside the submitted work. Juan M. Hincapie-Castillo reported grants from Merck outside the submitted work. Debbie Wilson reported grants from the National Institute on Drug Abuse, the National Institute on Aging, Merck Sharp & Dohme, and Bristol Myers Squibb outside the submitted work; and serving as an editorial board member for the Journal of Pharmacy Technology. Ching-Yuan Chang's contributions to this manuscript were made while at the University of Florida College of Pharmacy. Ching-Yuan Chang is currently employed by Vertex Pharmaceuticals, Inc. Vertex did not fund or have any involvement in this study or publication. Vakaramoko Diaby is currently employed by Otsuka, Inc. Otsuka did not fund or have any involvement in this study or publication. No other disclosures were reported.
Conflict of interest statement
Wei-Hsuan Lo-Ciganic reported grants from the National Institute on Drug Abuse (R01DA044985 and R01DA050676), the National Institute on Aging (R21AG060308), the National Institute of Mental Health (R01MH121907), Merck Sharp & Dohme, Bristol Myers Squibb, the Richard King Mellon Foundation at the University of Pittsburgh, the Clinical and Translational Science Institute of the University of Florida, the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation, and the US Department of Veterans Affairs outside the submitted work; in addition, Wei-Hsuan Lo-Ciganic has a patent pending for U1195.70174US00. Haesuk Park reported grants from Bristol Myers Squibb/Pfizer Alliance American Thrombosis Investigator Initiated Research Program (ARISTA-USA) outside the submitted work. Juan M. Hincapie-Castillo reported grants from Merck outside the submitted work. Debbie Wilson reported grants from the National Institute on Drug Abuse, the National Institute on Aging, Merck Sharp & Dohme, and Bristol Myers Squibb outside the submitted work; and serving as an editorial board member for the Journal of Pharmacy Technology. Ching-Yuan Chang’s contributions to this manuscript were made while at the University of Florida College of Pharmacy. Ching-Yuan Chang is currently employed by Vertex Pharmaceuticals, Inc. Vertex did not fund or have any involvement in this study or publication. Vakaramoko Diaby is currently employed by Otsuka, Inc. Otsuka did not fund or have any involvement in this study or publication. No other disclosures were reported.
Figures

Similar articles
-
Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition.Br J Psychiatry. 2019 Jan;214(1):27-35. doi: 10.1192/bjp.2018.257. Epub 2018 Dec 6. Br J Psychiatry. 2019. PMID: 30520709 Free PMC article.
-
Factors associated with medication nonadherence among Medicare low-income subsidy beneficiaries with diabetes, hypertension, and/or heart failure.J Manag Care Spec Pharm. 2021 Aug;27(8):971-981. doi: 10.18553/jmcp.2021.27.8.971. J Manag Care Spec Pharm. 2021. PMID: 34337985 Free PMC article.
-
Higher costs and therapeutic factors associated with adherence to NCQA HEDIS antidepressant medication management measures: analysis of administrative claims.J Manag Care Pharm. 2006 Jan-Feb;12(1):43-54. doi: 10.18553/jmcp.2006.12.1.43. J Manag Care Pharm. 2006. PMID: 16420107 Free PMC article.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Antidepressants for depression in adults with HIV infection.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD008525. doi: 10.1002/14651858.CD008525.pub3. Cochrane Database Syst Rev. 2018. PMID: 29355886 Free PMC article. Review.
Cited by
-
Racial and social inequities in medication use: A review of articles responding to the Journal of Managed Care + Specialty Pharmacy's Call to Action.J Manag Care Spec Pharm. 2024 Jul;30(7):736-746. doi: 10.18553/jmcp.2024.30.7.736. J Manag Care Spec Pharm. 2024. PMID: 38950161 Free PMC article. Review.
References
-
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. National Cancer Institute. Accessed February 1, 2021. https://seer.cancer.gov/statfacts/html/breast.html
-
- Mehnert A, Brahler E, Faller H, et al. . Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol. 2014;32(31):3540-6. doi:10.1200/JCO.2014.56.0086 - PubMed
-
- Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30(4):407-17. doi:10.3122/jabfm.2017.04.170112 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

