TRIM21 inhibits irradiation-induced mitochondrial DNA release and impairs antitumour immunity in nasopharyngeal carcinoma tumour models
- PMID: 36797289
- PMCID: PMC9935546
- DOI: 10.1038/s41467-023-36523-y
TRIM21 inhibits irradiation-induced mitochondrial DNA release and impairs antitumour immunity in nasopharyngeal carcinoma tumour models
Abstract
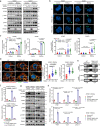
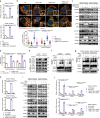
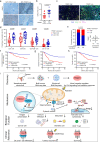
Although radiotherapy can promote antitumour immunity, the mechanisms underlying this phenomenon remain unclear. Here, we demonstrate that the expression of the E3 ubiquitin ligase, tumour cell-intrinsic tripartite motif-containing 21 (TRIM21) in tumours, is inversely associated with the response to radiation and CD8+ T cell-mediated antitumour immunity in nasopharyngeal carcinoma (NPC). Knockout of TRIM21 modulates the cGAS/STING cytosolic DNA sensing pathway, potentiates the antigen-presenting capacity of NPC cells, and activates cytotoxic T cell-mediated antitumour immunity in response to radiation. Mechanistically, TRIM21 promotes the degradation of the mitochondrial voltage-dependent anion-selective channel protein 2 (VDAC2) via K48-linked ubiquitination, which inhibits pore formation by VDAC2 oligomers for mitochondrial DNA (mtDNA) release, thereby inhibiting type-I interferon responses following radiation exposure. In patients with NPC, high TRIM21 expression was associated with poor prognosis and early tumour relapse after radiotherapy. Our findings reveal a critical role of TRIM21 in radiation-induced antitumour immunity, providing potential targets for improving the efficacy of radiotherapy in patients with NPC.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
USP44 regulates irradiation-induced DNA double-strand break repair and suppresses tumorigenesis in nasopharyngeal carcinoma.Nat Commun. 2022 Jan 25;13(1):501. doi: 10.1038/s41467-022-28158-2. Nat Commun. 2022. PMID: 35079021 Free PMC article.
-
FGF19 promotes nasopharyngeal carcinoma progression by inducing angiogenesis via inhibiting TRIM21-mediated ANXA2 ubiquitination.Cell Oncol (Dordr). 2024 Feb;47(1):283-301. doi: 10.1007/s13402-023-00868-9. Epub 2023 Oct 2. Cell Oncol (Dordr). 2024. PMID: 37782406 Free PMC article.
-
TRIM21-SERPINB5 aids GMPS repression to protect nasopharyngeal carcinoma cells from radiation-induced apoptosis.J Biomed Sci. 2020 Jan 31;27(1):30. doi: 10.1186/s12929-020-0625-7. J Biomed Sci. 2020. PMID: 32005234 Free PMC article.
-
Radio-Susceptibility of Nasopharyngeal Carcinoma: Focus on Epstein- Barr Virus, MicroRNAs, Long Non-Coding RNAs and Circular RNAs.Curr Mol Pharmacol. 2020;13(3):192-205. doi: 10.2174/1874467213666191227104646. Curr Mol Pharmacol. 2020. PMID: 31880267 Review.
-
A Interacting Model: How TRIM21 Orchestrates with Proteins in Intracellular Immunity.Small Methods. 2024 Jan;8(1):e2301142. doi: 10.1002/smtd.202301142. Epub 2023 Nov 3. Small Methods. 2024. PMID: 37922533 Review.
Cited by
-
MDM4 inhibits ferroptosis in p53 mutant colon cancer via regulating TRIM21/GPX4 expression.Cell Death Dis. 2024 Nov 14;15(11):825. doi: 10.1038/s41419-024-07227-y. Cell Death Dis. 2024. PMID: 39543140 Free PMC article.
-
The circadian gene ARNTL2 promotes nasopharyngeal carcinoma invasiveness and metastasis through suppressing AMOTL2-LATS-YAP pathway.Cell Death Dis. 2024 Jul 2;15(7):466. doi: 10.1038/s41419-024-06860-x. Cell Death Dis. 2024. PMID: 38956029 Free PMC article.
-
E2F3 renders an immunosuppressive tumor microenvironment in nasopharyngeal carcinoma: Involvements of the transcription activation of PRC1 and BIRC5.Immun Inflamm Dis. 2023 Aug;11(8):e987. doi: 10.1002/iid3.987. Immun Inflamm Dis. 2023. PMID: 37647439 Free PMC article.
-
PJA1-mediated suppression of pyroptosis as a driver of docetaxel resistance in nasopharyngeal carcinoma.Nat Commun. 2024 Jun 21;15(1):5300. doi: 10.1038/s41467-024-49675-2. Nat Commun. 2024. PMID: 38906860 Free PMC article.
-
SRplot: A free online platform for data visualization and graphing.PLoS One. 2023 Nov 9;18(11):e0294236. doi: 10.1371/journal.pone.0294236. eCollection 2023. PLoS One. 2023. PMID: 37943830 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

