AIM2 and Psoriasis
- PMID: 36742336
- PMCID: PMC9889639
- DOI: 10.3389/fimmu.2023.1085448
AIM2 and Psoriasis
Abstract
Psoriasis is a chronic inflammatory skin disease occurring worldwide, with multiple systemic complications, which seriously affect the quality of life and physical and mental health of patients. The pathogenesis of psoriasis is related to the environment, genetics, epigenetics, and dysregulation of immune cells such as T cells, dendritic cells (DCs), and nonimmune cells such as keratinocytes. Absent in melanoma 2 (AIM2), a susceptibility gene locus for psoriasis, has been strongly linked to the genetic and epigenetic aspects of psoriasis and increased in expression in psoriatic keratinocytes. AIM2 was found to be activated in an inflammasome-dependent way to release IL-1β and IL-18 to mediate inflammation, and to participate in immune regulation in psoriasis, or in an inflammasome-independent way by regulating the function of regulatory T(Treg) cells or programming cell death in keratinocytes as well as controlling the proliferative state of different cells. AIM2 may also play a role in the recurrence of psoriasis by trained immunity. In this review, we will elaborate on the characteristics of AIM2 and how AIM2 mediates the development of psoriasis.
Keywords: AIM2; epigenetic; inflammasome; keratinocytes; psoriasis; trained immunity.
Copyright © 2023 Zhang, Xu, Cheng and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

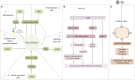
Similar articles
-
Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions.Sci Transl Med. 2011 May 11;3(82):82ra38. doi: 10.1126/scitranslmed.3002001. Sci Transl Med. 2011. PMID: 21562230 Free PMC article.
-
Neutrophil extracellular traps promote keratinocyte inflammation via AIM2 inflammasome and AIM2-XIAP in psoriasis.Exp Dermatol. 2023 Apr;32(4):368-378. doi: 10.1111/exd.14711. Epub 2022 Nov 23. Exp Dermatol. 2023. PMID: 36401800
-
EFLA 945 restricts AIM2 inflammasome activation by preventing DNA entry for psoriasis treatment.Cytokine. 2020 Mar;127:154951. doi: 10.1016/j.cyto.2019.154951. Epub 2019 Dec 11. Cytokine. 2020. PMID: 31837587
-
Roles of AIM2 Gene and AIM2 Inflammasome in the Pathogenesis and Treatment of Psoriasis.Front Genet. 2022 Sep 2;13:929162. doi: 10.3389/fgene.2022.929162. eCollection 2022. Front Genet. 2022. PMID: 36118867 Free PMC article. Review.
-
The Role of NLRP1, NLRP3, and AIM2 Inflammasomes in Psoriasis: Review.Int J Mol Sci. 2021 May 31;22(11):5898. doi: 10.3390/ijms22115898. Int J Mol Sci. 2021. PMID: 34072753 Free PMC article. Review.
Cited by
-
Pyroptosis: a new insight into intestinal inflammation and cancer.Front Immunol. 2024 Feb 22;15:1364911. doi: 10.3389/fimmu.2024.1364911. eCollection 2024. Front Immunol. 2024. PMID: 38455052 Free PMC article. Review.
-
Intracellular DNA sensing by neutrophils and amplification of the innate immune response.Front Immunol. 2023 Jul 7;14:1208137. doi: 10.3389/fimmu.2023.1208137. eCollection 2023. Front Immunol. 2023. PMID: 37483598 Free PMC article. Review.
-
Exploring the Common Pathogenic Mechanisms of Psoriasis and Atopic Dermatitis: The Interaction between SGK1 and TIGIT Signaling Pathways.Inflammation. 2024 Aug 1. doi: 10.1007/s10753-024-02115-1. Online ahead of print. Inflammation. 2024. PMID: 39088121
-
Epigenetic Regulation in Oral Squamous Cell Carcinoma Microenvironment: A Comprehensive Review.Cancers (Basel). 2023 Nov 27;15(23):5600. doi: 10.3390/cancers15235600. Cancers (Basel). 2023. PMID: 38067304 Free PMC article. Review.
-
Identification of neutrophil extracellular traps genes as potential biomarkers in psoriasis based on bioinformatics analysis.Sci Rep. 2024 Oct 11;14(1):23848. doi: 10.1038/s41598-024-75069-x. Sci Rep. 2024. PMID: 39394253 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

