Design of Nanohydrogels for Targeted Intracellular Drug Transport to the Trans-Golgi Network
- PMID: 36739269
- PMCID: PMC11469190
- DOI: 10.1002/adhm.202201794
Design of Nanohydrogels for Targeted Intracellular Drug Transport to the Trans-Golgi Network
Abstract
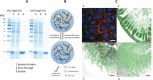
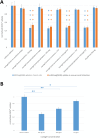
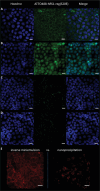
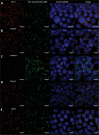
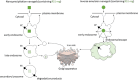
Nanohydrogels combine advantages of hydrogels and nanoparticles. In particular, they represent promising drug delivery systems. Nanogel synthesis by oxidative condensation of polyglycidol prepolymers, that are modified with thiol groups, results in crosslinking by disulfide bonds. Hereby, biomolecules like the antidiabetic peptide RS1-reg, derived from the regulatory protein RS1 of the Na+ -D-glucose cotransporter SGLT1, can be covalently bound by cysteine residues to the nanogel in a hydrophilic, stabilizing environment. After oral uptake, the acid-stable nanogels protect their loading during gastric passage from proteolytic degradation. Under alkaline conditions in small intestine the nanohydrogels become mucoadhesive, pass the intestinal mucosa and are taken up into small intestinal enterocytes by endocytosis. Using Caco-2 cells as a model for small intestinal enterocytes, by confocal laser scanning microscopy and structured illumination microscopy, the colocalization of fluorescent-labeled RS1-reg with markers of endosomes, lysosomes, and trans-Golgi-network after uptake with polyglycidol-based nanogels formed by precipitation polymerization is demonstrated. This indicates that RS1-reg follows the endosomal pathway. In the following, the design of bespoken nanohydrogels for specific targeting of RS1-reg to its site of action at the trans-Golgi network is described that might also represent a way of targeted transport for other drugs to their targets at the Golgi apparatus.
Keywords: drug delivery; nanohydrogels; regulation of the Na+-D-glucose cotransporter SGLT1 in intestine; regulatory protein RS1; targeted transport.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A Modified Tripeptide Motif of RS1 (RSC1A1) Down-Regulates Exocytotic Pathways of Human Na+-d-glucose Cotransporters SGLT1, SGLT2, and Glucose Sensor SGLT3 in the Presence of Glucose.Mol Pharmacol. 2019 Jan;95(1):82-96. doi: 10.1124/mol.118.113514. Epub 2018 Oct 24. Mol Pharmacol. 2019. PMID: 30355744
-
Protein RS1 (RSC1A1) Downregulates the Exocytotic Pathway of Glucose Transporter SGLT1 at Low Intracellular Glucose via Inhibition of Ornithine Decarboxylase.Mol Pharmacol. 2016 Nov;90(5):508-521. doi: 10.1124/mol.116.104521. Epub 2016 Aug 23. Mol Pharmacol. 2016. PMID: 27555600
-
Tripeptides of RS1 (RSC1A1) inhibit a monosaccharide-dependent exocytotic pathway of Na+-D-glucose cotransporter SGLT1 with high affinity.J Biol Chem. 2007 Sep 28;282(39):28501-28513. doi: 10.1074/jbc.M705416200. Epub 2007 Aug 8. J Biol Chem. 2007. PMID: 17686765
-
Function and presumed molecular structure of Na(+)-D-glucose cotransport systems.J Membr Biol. 1994 Feb;138(1):1-11. doi: 10.1007/BF00211064. J Membr Biol. 1994. PMID: 8189427 Review.
-
Regulation of Na+/glucose cotransporters.J Exp Biol. 1997 Jan;200(Pt 2):287-93. doi: 10.1242/jeb.200.2.287. J Exp Biol. 1997. PMID: 9050236 Review.
References
-
- Peppas N. A., Bures P., Leobandung W., Ichikawa H., Eur. J. Pharm. Biopharm. 2000, 50, 27. - PubMed
-
- Kamath K. R., Park K., Adv. Drug Delivery Rev. 1993, 11, 59.
-
- Kim S. W., Bae Y. H., Okano T., Pharm Res 1992, 9, 283. - PubMed
-
- Schwall C. T., Banerjee I. A., Materials 2009, 2, 577.
-
- Cuggino J. C., Blanco E. R. O., Gugliotta L. M., Igarzabal C. I. A., Calderón M., J. Controlled Release 2019, 307, 221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

