Lymphotoxin beta receptor signaling directly controls airway smooth muscle deregulation and asthmatic lung dysfunction
- PMID: 36473503
- PMCID: PMC10081945
- DOI: 10.1016/j.jaci.2022.11.016
Lymphotoxin beta receptor signaling directly controls airway smooth muscle deregulation and asthmatic lung dysfunction
Abstract
Background: Dysregulation of airway smooth muscle cells (ASM) is central to the severity of asthma. Which molecules dominantly control ASM in asthma is unclear. High levels of the cytokine LIGHT (aka TNFSF14) have been linked to asthma severity and lower baseline predicted FEV1 percentage, implying that signals through its receptors might directly control ASM dysfunction.
Objective: Our study sought to determine whether signaling via lymphotoxin beta receptor (LTβR) or herpesvirus entry mediator from LIGHT dominantly drives ASM hyperreactivity induced by allergen.
Methods: Conditional knockout mice deficient for LTβR or herpesvirus entry mediator in smooth muscle cells were used to determine their role in ASM deregulation and airway hyperresponsiveness (AHR) in vivo. Human ASM were used to study signals induced by LTβR.
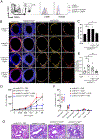
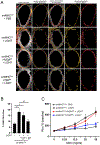
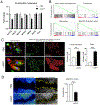
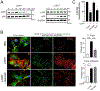
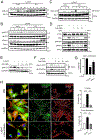
Results: LTβR was strongly expressed in ASM from normal and asthmatic subjects compared to several other receptors implicated in smooth muscle deregulation. Correspondingly, conditional deletion of LTβR only in smooth muscle cells in smMHCCreLTβRfl/fl mice minimized changes in their numbers and mass as well as AHR induced by house dust mite allergen in a model of severe asthma. Intratracheal LIGHT administration independently induced ASM hypertrophy and AHR in vivo dependent on direct LTβR signals to ASM. LIGHT promoted contractility, hypertrophy, and hyperplasia of human ASM in vitro. Distinguishing LTβR from the receptors for IL-13, TNF, and IL-17, which have also been implicated in smooth muscle dysregulation, LIGHT promoted NF-κB-inducing kinase-dependent noncanonical nuclear factor kappa-light-chain enhancer of activated B cells in ASM in vitro, leading to sustained accumulation of F-actin, phosphorylation of myosin light chain kinase, and contractile activity.
Conclusions: LTβR signals directly and dominantly drive airway smooth muscle hyperresponsiveness relevant for pathogenesis of airway remodeling in severe asthma.
Keywords: AHR; LIGHT; LTβR; TNF superfamily; TNFSF14; airway smooth muscle; asthma; contractility; noncanonical NF-κB.
Copyright © 2022 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

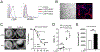
Similar articles
-
Lymphotoxin β receptor signaling induces IL-8 production in human bronchial epithelial cells.PLoS One. 2014 Dec 11;9(12):e114791. doi: 10.1371/journal.pone.0114791. eCollection 2014. PLoS One. 2014. PMID: 25501580 Free PMC article.
-
Smooth muscle CaMKIIδ promotes allergen-induced airway hyperresponsiveness and inflammation.Pflugers Arch. 2015 Dec;467(12):2541-54. doi: 10.1007/s00424-015-1713-5. Epub 2015 Jun 20. Pflugers Arch. 2015. PMID: 26089028 Free PMC article.
-
Smooth muscle brain-derived neurotrophic factor contributes to airway hyperreactivity in a mouse model of allergic asthma.FASEB J. 2019 Feb;33(2):3024-3034. doi: 10.1096/fj.201801002R. Epub 2018 Oct 23. FASEB J. 2019. PMID: 30351991 Free PMC article.
-
The contribution of airway smooth muscle to airway narrowing and airway hyperresponsiveness in disease.Eur Respir J. 2000 Aug;16(2):349-54. doi: 10.1034/j.1399-3003.2000.16b25.x. Eur Respir J. 2000. PMID: 10968513 Review.
-
The Impact of Monoclonal Antibodies on Airway Smooth Muscle Contractility in Asthma: A Systematic Review.Biomedicines. 2021 Sep 21;9(9):1281. doi: 10.3390/biomedicines9091281. Biomedicines. 2021. PMID: 34572466 Free PMC article. Review.
Cited by
-
TRPV4 Activation during Guinea Pig Airway Smooth Muscle Contraction Promotes Ca2+ and Na+ Influx.Pharmaceuticals (Basel). 2024 Feb 24;17(3):293. doi: 10.3390/ph17030293. Pharmaceuticals (Basel). 2024. PMID: 38543079 Free PMC article.
-
Muscular hyperplasia in Crohn's disease strictures: through thick and thin.Am J Physiol Cell Physiol. 2024 Sep 1;327(3):C671-C683. doi: 10.1152/ajpcell.00307.2024. Epub 2024 Jun 24. Am J Physiol Cell Physiol. 2024. PMID: 38912732 Review.
-
Exploring potential therapeutic targets for asthma: a proteome-wide Mendelian randomization analysis.J Transl Med. 2024 Oct 29;22(1):978. doi: 10.1186/s12967-024-05782-8. J Transl Med. 2024. PMID: 39472987 Free PMC article.
-
The key piece of the airway remodeling puzzle revealed-LIGHT signaling in the lung.Genes Dis. 2023 Mar 27;10(4):1131-1132. doi: 10.1016/j.gendis.2023.02.021. eCollection 2023 Jul. Genes Dis. 2023. PMID: 37397546 Free PMC article. No abstract available.
References
-
- Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 2001; 344:350–62. - PubMed
-
- Zuyderduyn S, Sukkar MB, Fust A, Dhaliwal S, Burgess JK. Treating asthma means treating airway smooth muscle cells. Eur Respir J 2008; 32:265–74. - PubMed
-
- Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, et al. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol 2005; 116:544–9. - PubMed
-
- Kaminska M, Foley S, Maghni K, Storness-Bliss C, Coxson H, Ghezzo H, et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J Allergy Clin Immunol 2009; 124:45–51. e1–4. - PubMed
-
- Mahn K, Ojo OO, Chadwick G, Aaronson PI, Ward JP, Lee TH. Ca(2+) homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax 2010; 65:547–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

