Cannabinoid non-cannabidiol site modulation of TRPV2 structure and function
- PMID: 36470868
- PMCID: PMC9722916
- DOI: 10.1038/s41467-022-35163-y
Cannabinoid non-cannabidiol site modulation of TRPV2 structure and function
Abstract
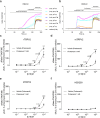
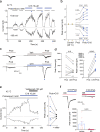
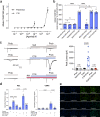
TRPV2 is a ligand-operated temperature sensor with poorly defined pharmacology. Here, we combine calcium imaging and patch-clamp electrophysiology with cryo-electron microscopy (cryo-EM) to explore how TRPV2 activity is modulated by the phytocannabinoid Δ9-tetrahydrocannabiorcol (C16) and by probenecid. C16 and probenecid act in concert to stimulate TRPV2 responses including histamine release from rat and human mast cells. Each ligand causes distinct conformational changes in TRPV2 as revealed by cryo-EM. Although the binding for probenecid remains elusive, C16 associates within the vanilloid pocket. As such, the C16 binding location is distinct from that of cannabidiol, partially overlapping with the binding site of the TRPV2 inhibitor piperlongumine. Taken together, we discover a new cannabinoid binding site in TRPV2 that is under the influence of allosteric control by probenecid. This molecular insight into ligand modulation enhances our understanding of TRPV2 in normal and pathophysiology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



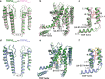


Similar articles
-
Cannabidiol sensitizes TRPV2 channels to activation by 2-APB.Elife. 2023 May 18;12:e86166. doi: 10.7554/eLife.86166. Elife. 2023. PMID: 37199723 Free PMC article.
-
Molecular mechanism of TRPV2 channel modulation by cannabidiol.Elife. 2019 Sep 30;8:e48792. doi: 10.7554/eLife.48792. Elife. 2019. PMID: 31566564 Free PMC article.
-
Cryo-electron microscopy structure of the TRPV2 ion channel.Nat Struct Mol Biol. 2016 Feb;23(2):180-186. doi: 10.1038/nsmb.3159. Epub 2016 Jan 18. Nat Struct Mol Biol. 2016. PMID: 26779611 Free PMC article.
-
What do we know about the transient receptor potential vanilloid 2 (TRPV2) ion channel?FEBS J. 2013 Nov;280(21):5471-87. doi: 10.1111/febs.12302. Epub 2013 May 28. FEBS J. 2013. PMID: 23615321 Free PMC article. Review.
-
Determining the Crystal Structure of TRPV6.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review.
Cited by
-
TRPV2: a universal regulator in cellular physiology with a yet poorly defined thermosensitivity.J Physiol Sci. 2024 Sep 16;74(1):42. doi: 10.1186/s12576-024-00936-1. J Physiol Sci. 2024. PMID: 39285320 Free PMC article. Review.
-
The Role of TRP Channels in Sepsis and Colitis.Int J Mol Sci. 2024 Apr 27;25(9):4784. doi: 10.3390/ijms25094784. Int J Mol Sci. 2024. PMID: 38731999 Free PMC article. Review.
-
Tyrosine phosphorylation and palmitoylation of TRPV2 ion channel tune microglial beta-amyloid peptide phagocytosis.J Neuroinflammation. 2024 Sep 3;21(1):218. doi: 10.1186/s12974-024-03204-6. J Neuroinflammation. 2024. PMID: 39227967 Free PMC article.
-
Cannabidiol sensitizes TRPV2 channels to activation by 2-APB.bioRxiv [Preprint]. 2023 Jan 27:2023.01.27.525817. doi: 10.1101/2023.01.27.525817. bioRxiv. 2023. Update in: Elife. 2023 May 18;12:e86166. doi: 10.7554/eLife.86166 PMID: 36747846 Free PMC article. Updated. Preprint.
-
Cannabidiol sensitizes TRPV2 channels to activation by 2-APB.Elife. 2023 May 18;12:e86166. doi: 10.7554/eLife.86166. Elife. 2023. PMID: 37199723 Free PMC article.
References
-
- Axelsson HE, et al. Transient receptor potential vanilloid 1, vanilloid 2 and melastatin 8 immunoreactive nerve fibers in human skin from individuals with and without Norrbottnian congenital insensitivity to pain. Neuroscience. 2009;162:1322–1332. doi: 10.1016/j.neuroscience.2009.05.052. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

