Vaccination induces HIV broadly neutralizing antibody precursors in humans
- PMID: 36454825
- PMCID: PMC11103259
- DOI: 10.1126/science.add6502
Vaccination induces HIV broadly neutralizing antibody precursors in humans
Abstract
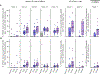
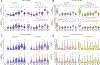
Broadly neutralizing antibodies (bnAbs) can protect against HIV infection but have not been induced by human vaccination. A key barrier to bnAb induction is vaccine priming of rare bnAb-precursor B cells. In a randomized, double-blind, placebo-controlled phase 1 clinical trial, the HIV vaccine-priming candidate eOD-GT8 60mer adjuvanted with AS01B had a favorable safety profile and induced VRC01-class bnAb precursors in 97% of vaccine recipients with median frequencies reaching 0.1% among immunoglobulin G B cells in blood. bnAb precursors shared properties with bnAbs and gained somatic hypermutation and affinity with the boost. The results establish clinical proof of concept for germline-targeting vaccine priming, support development of boosting regimens to induce bnAbs, and encourage application of the germline-targeting strategy to other targets in HIV and other pathogens.
Conflict of interest statement
Figures




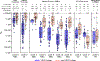

Comment in
-
Triggering rare HIV antibodies by vaccination.Science. 2022 Dec 2;378(6623):949-950. doi: 10.1126/science.adf3722. Epub 2022 Dec 1. Science. 2022. PMID: 36454831
Similar articles
-
Heterologous prime-boost vaccination drives early maturation of HIV broadly neutralizing antibody precursors in humanized mice.Sci Transl Med. 2024 May 22;16(748):eadn0223. doi: 10.1126/scitranslmed.adn0223. Epub 2024 May 22. Sci Transl Med. 2024. PMID: 38753806 Free PMC article.
-
Vaccine priming of rare HIV broadly neutralizing antibody precursors in nonhuman primates.Science. 2024 May 17;384(6697):eadj8321. doi: 10.1126/science.adj8321. Epub 2024 May 17. Science. 2024. PMID: 38753769 Free PMC article.
-
Humanized V(D)J-rearranging and TdT-expressing mouse vaccine models with physiological HIV-1 broadly neutralizing antibody precursors.Proc Natl Acad Sci U S A. 2023 Jan 3;120(1):e2217883120. doi: 10.1073/pnas.2217883120. Epub 2022 Dec 27. Proc Natl Acad Sci U S A. 2023. PMID: 36574685 Free PMC article.
-
Clinical development of SpikoGen®, an Advax-CpG55.2 adjuvanted recombinant spike protein vaccine.Hum Vaccin Immunother. 2024 Dec 31;20(1):2363016. doi: 10.1080/21645515.2024.2363016. Epub 2024 Jun 5. Hum Vaccin Immunother. 2024. PMID: 38839044 Free PMC article. Review.
-
Interventions for supporting pregnant women's decision-making about mode of birth after a caesarean.Cochrane Database Syst Rev. 2013 Jul 30;2013(7):CD010041. doi: 10.1002/14651858.CD010041.pub2. Cochrane Database Syst Rev. 2013. PMID: 23897547 Free PMC article. Review.
Cited by
-
A remarkable genetic shift in a transmitted/founder virus broadens antibody responses against HIV-1.Elife. 2024 Apr 15;13:RP92379. doi: 10.7554/eLife.92379. Elife. 2024. PMID: 38619110 Free PMC article.
-
Adaptive immune receptor genotyping using the corecount program.Front Immunol. 2023 Apr 11;14:1125884. doi: 10.3389/fimmu.2023.1125884. eCollection 2023. Front Immunol. 2023. PMID: 37114042 Free PMC article.
-
Exploring synergies between B- and T-cell vaccine approaches to optimize immune responses against HIV-workshop report.NPJ Vaccines. 2024 Feb 21;9(1):39. doi: 10.1038/s41541-024-00818-y. NPJ Vaccines. 2024. PMID: 38383616 Free PMC article.
-
Human Immunodeficiency Virus Vaccine: Promise and Challenges.Infect Dis Clin North Am. 2024 Sep;38(3):475-485. doi: 10.1016/j.idc.2024.04.004. Epub 2024 Jun 13. Infect Dis Clin North Am. 2024. PMID: 38876903 Review.
-
Perceptions and Acceptance of a Prophylactic Vaccine for Human Immunodeficiency Virus (HIV): A Qualitative Study.Patient. 2024 Jul;17(4):457-469. doi: 10.1007/s40271-024-00686-7. Epub 2024 Apr 6. Patient. 2024. PMID: 38581599 Free PMC article.
References
-
- Fauci AS, An HIV Vaccine Is Essential for Ending the HIV/AIDS Pandemic. JAMA 318, 1535–1536 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

