Evaluation of Adjuvant Activity and Bio-Distribution of Archaeosomes Prepared Using Microfluidic Technology
- PMID: 36365110
- PMCID: PMC9697222
- DOI: 10.3390/pharmaceutics14112291
Evaluation of Adjuvant Activity and Bio-Distribution of Archaeosomes Prepared Using Microfluidic Technology
Abstract
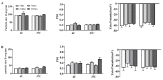
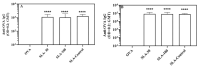
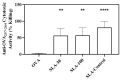
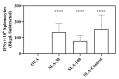
Archaeosomes, composed of sulfated lactosyl archaeol (SLA) glycolipids, have been proven to be an effective vaccine adjuvant in multiple preclinical models of infectious disease or cancer. They have classically been prepared using a thin-film hydration method with an average particle size of 100-200 nm. In this study, we developed methods to generate SLA archaeosomes at different sizes, i.e., 30 nm and 100 nm, via microfluidic mixing technology and evaluated their physicochemical characteristics, as well as adjuvant activity and in vivo biodistribution in mice. Archaeosomes, prepared using thin-film and microfluidic mixing techniques, had similar nanostructures and physicochemical characteristics, with both appearing stable during the course of this study when stored at 4 °C or 37 °C. They also demonstrated similar adjuvant activity when admixed with ovalbumin antigen and used to immunize mice, generating equivalent antigen-specific immune responses. Archaeosomes, labeled with CellVueTM NIR815, had an equivalent biodistribution with both sizes, namely the highest signal at the injection site at 24 h post injection, followed by liver, spleen and inguinal lymph node. The presence of SLA archaeosomes of either size helped to retain OVA antigen (OVA-Cy5.5) longer at the injection site than unadjuvanted OVA. Overall, archaeosomes of two sizes (30 nm and 100 nm) prepared using microfluidic mixing maintained similar physicochemical properties, adjuvant activity and biodistribution of antigen, in comparison to those compared by the conventional thin film hydration method. This suggests that microfluidics based approaches could be applied to generate consistently sized archaeosomes for use as a vaccine adjuvant.
Keywords: archaeosome; bio-distribution; glycolipid; liposome; microfluidics; sulfated lactosyl archaeol; vaccine adjuvant.
Conflict of interest statement
Yimei Jia, Bassel Akache, Lakshmi Krishnan and Michael McCluskie are inventors on a SLA archaeosome-related patent application. All other authors have no competing/conflicts of interest.
Figures

Similar articles
-
The Synergistic Effects of Sulfated Lactosyl Archaeol Archaeosomes When Combined with Different Adjuvants in a Murine Model.Pharmaceutics. 2021 Feb 2;13(2):205. doi: 10.3390/pharmaceutics13020205. Pharmaceutics. 2021. PMID: 33540932 Free PMC article.
-
Assessment of stability of sulphated lactosyl archaeol archaeosomes for use as a vaccine adjuvant.J Liposome Res. 2021 Sep;31(3):237-245. doi: 10.1080/08982104.2020.1786115. Epub 2020 Jul 23. J Liposome Res. 2021. PMID: 32583693
-
A comparison of the immune responses induced by antigens in three different archaeosome-based vaccine formulations.Int J Pharm. 2019 Apr 20;561:187-196. doi: 10.1016/j.ijpharm.2019.02.041. Epub 2019 Mar 2. Int J Pharm. 2019. PMID: 30836154
-
Archaeobacterial ether lipid liposomes (archaeosomes) as novel vaccine and drug delivery systems.Crit Rev Biotechnol. 1999;19(4):317-57. doi: 10.1080/0738-859991229170. Crit Rev Biotechnol. 1999. PMID: 10723627 Review.
-
Archaeosome immunostimulatory vaccine delivery system.Curr Drug Deliv. 2005 Oct;2(4):407-21. doi: 10.2174/156720105774370285. Curr Drug Deliv. 2005. PMID: 16305444 Review.
Cited by
-
Sulfated lactosyl archaeol (SLA) archaeosomes as a vaccine adjuvant.Hum Vaccin Immunother. 2024 Dec 31;20(1):2395081. doi: 10.1080/21645515.2024.2395081. Epub 2024 Sep 15. Hum Vaccin Immunother. 2024. PMID: 39278862 Free PMC article. Review.
-
Tetraether archaeal lipids promote long-term survival in extreme conditions.Mol Microbiol. 2024 May;121(5):882-894. doi: 10.1111/mmi.15240. Epub 2024 Feb 19. Mol Microbiol. 2024. PMID: 38372181
-
Archaeosomes for Oral Drug Delivery: From Continuous Microfluidics Production to Powdered Formulations.Pharmaceutics. 2024 May 23;16(6):694. doi: 10.3390/pharmaceutics16060694. Pharmaceutics. 2024. PMID: 38931818 Free PMC article.
References
-
- Akache B., Stark F.C., Jia Y., Deschatelets L., Dudani R., Harrison B.A., Agbayani G., Williams D., Jamshidi M.P., Krishnan L., et al. Sulfated archaeol glycolipids: Comparison with other immunological adjuvants in mice. PLoS ONE. 2018;13:e0208067. doi: 10.1371/journal.pone.0208067. - DOI - PMC - PubMed
-
- Perera D.J., Hassan A.S., Jia Y., Ricciardi A., McCluskie M.J., Weeratna R.D., Ndao M. Adjuvanted Schistosoma mansoni-Cathepsin B with Sulfated Lactosyl Archaeol Archaeosomes or AddaVax™ Provide Protection in a Pre-Clinical Schistosomiasis Model. Front. Immunol. 2020;11:605288. doi: 10.3389/fimmu.2020.605288. - DOI - PMC - PubMed
-
- Akache B., Deschatelets L., Harrison B.A., Dudani R., Stark F.C., Jia Y., Landi A., Law J.L.M., Logan M., Hockman D., et al. Effect of Different Adjuvants on the Longevity and Strength of Humoral and Cellular Immune Responses to the HCV Envelope Glycoproteins. Vaccines. 2019;7:204. doi: 10.3390/vaccines7040204. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

