The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction
- PMID: 36357415
- PMCID: PMC9649722
- DOI: 10.1038/s41467-022-34403-5
The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction
Abstract
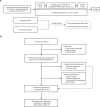
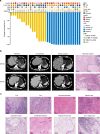
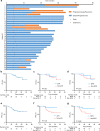
The synergistic effect of neoadjuvant immunotherapy and chemoradiotherapy in gastric adenocarcinoma is unclear. This phase II trial (NCT03631615) investigated this neoadjuvant combination in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Thirty-six patients received capecitabine 850 mg/m2 twice daily and simultaneous radiotherapy for 5 weeks, sandwiched by a 21-day cycle of oxaliplatin 130 mg/m2 (day 1) plus capecitabine 1000 mg/m2 twice daily (days 1-14), respectively, followed by surgery. Camrelizumab 200 mg (day 1) was given for 5 cycles since initiating chemotherapy. Primary endpoint was pathological complete response (pCR, ypT0) rate. Secondary endpoints included total pCR (tpCR, ypT0N0) rate, major pathological response (MPR, < 10% residual tumor cells) rate, margin-free (R0) resection rate, downstaging, progression-free survival (PFS), overall survival (OS), and safety. The pCR rate was 33.3% (95% CI, 18.6-51.0), meeting pre-specified endpoint. TpCR, MPR, and R0 resection rates were 33.3%, 44.4%, and 91.7%, respectively. Twenty-eight (77.8%) patients reached ypN0. Two-year PFS and OS rates were 66.9% and 76.1%, respectively. The most common grade 3-4 adverse event was decreased lymphocyte count (27 [75.0%]). Neoadjuvant camrelizumab plus concurrent chemoradiotherapy exhibits promising pathological response in patients with locally advanced gastric adenocarcinoma, with an acceptable safety profile.
© 2022. The Author(s).
Conflict of interest statement
Rongrong Zheng and Zhiguo Hou are employees of Jiangsu Hengrui Pharmaceuticals Co., Ltd. The remaining authors declare no competing interests.
Figures
Similar articles
-
Efficacy and safety of camrelizumab combined with oxaliplatin and S-1 as neoadjuvant treatment in locally advanced gastric or gastroesophageal junction cancer: A phase II, single-arm study.Cancer Med. 2024 Feb;13(3):e7006. doi: 10.1002/cam4.7006. Cancer Med. 2024. PMID: 38400680 Free PMC article. Clinical Trial.
-
Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study.J Immunother Cancer. 2022 Mar;10(3):e003635. doi: 10.1136/jitc-2021-003635. J Immunother Cancer. 2022. PMID: 35296556 Free PMC article. Clinical Trial.
-
Neoadjuvant short-course radiotherapy followed by camrelizumab and chemotherapy in locally advanced rectal cancer (UNION): early outcomes of a multicenter randomized phase III trial.Ann Oncol. 2024 Oct;35(10):882-891. doi: 10.1016/j.annonc.2024.06.015. Epub 2024 Jul 2. Ann Oncol. 2024. PMID: 38964714 Clinical Trial.
-
Galunisertib plus neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a single-arm, phase 2 trial.Lancet Oncol. 2022 Sep;23(9):1189-1200. doi: 10.1016/S1470-2045(22)00446-6. Epub 2022 Aug 8. Lancet Oncol. 2022. PMID: 35952709 Clinical Trial.
-
Combination of immune checkpoint inhibitors and radiotherapy in locally advanced esophagogastric junction adenocarcinoma: A review.Cancer. 2024 Dec 1;130(23):4040-4051. doi: 10.1002/cncr.35561. Epub 2024 Sep 19. Cancer. 2024. PMID: 39297403 Review.
Cited by
-
Development and validation of a nomogram to predict pathological complete response in patients with locally advanced gastric adenocarcinoma treated with neoadjuvant chemotherapy in combination with PD-1 antibodies.J Gastrointest Oncol. 2023 Dec 31;14(6):2373-2383. doi: 10.21037/jgo-23-751. Epub 2023 Dec 8. J Gastrointest Oncol. 2023. PMID: 38196541 Free PMC article.
-
Unveiling promising targets in gastric cancer therapy: A comprehensive review.Mol Ther Oncol. 2024 Aug 5;32(3):200857. doi: 10.1016/j.omton.2024.200857. eCollection 2024 Sep 19. Mol Ther Oncol. 2024. PMID: 39280587 Free PMC article. Review.
-
Neoadjuvant Immunotherapy: A Promising New Standard of Care.Int J Mol Sci. 2023 Jul 24;24(14):11849. doi: 10.3390/ijms241411849. Int J Mol Sci. 2023. PMID: 37511609 Free PMC article. Review.
-
Neoadjuvant sintilimab in combination with concurrent chemoradiotherapy for locally advanced gastric or gastroesophageal junction adenocarcinoma: a single-arm phase 2 trial.Nat Commun. 2023 Aug 14;14(1):4904. doi: 10.1038/s41467-023-40480-x. Nat Commun. 2023. PMID: 37580320 Free PMC article. Clinical Trial.
-
Comparison of neoadjuvant immunotherapy versus routine neoadjuvant therapy for patients with locally advanced esophageal cancer: A systematic review and meta-analysis.Front Immunol. 2023 Mar 23;14:1108213. doi: 10.3389/fimmu.2023.1108213. eCollection 2023. Front Immunol. 2023. PMID: 37033991 Free PMC article.
References
-
- National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology. gastric cancer, version 5. https://www.nccn.org/professionals/physician_gls/default.aspx (2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

