The coilin N-terminus mediates multivalent interactions between coilin and Nopp140 to form and maintain Cajal bodies
- PMID: 36224177
- PMCID: PMC9556525
- DOI: 10.1038/s41467-022-33434-2
The coilin N-terminus mediates multivalent interactions between coilin and Nopp140 to form and maintain Cajal bodies
Abstract
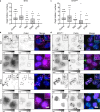
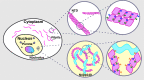
Cajal bodies (CBs) are ubiquitous nuclear membraneless organelles (MLOs) that concentrate and promote efficient biogenesis of snRNA-protein complexes involved in splicing (snRNPs). Depletion of the CB scaffolding protein coilin disperses snRNPs, making CBs a model system for studying the structure and function of MLOs. Although it is assumed that CBs form through condensation, the biomolecular interactions responsible remain elusive. Here, we discover the unexpected capacity of coilin's N-terminal domain (NTD) to form extensive fibrils in the cytoplasm and discrete nuclear puncta in vivo. Single amino acid mutational analysis reveals distinct molecular interactions between coilin NTD proteins to form fibrils and additional NTD interactions with the nuclear Nopp140 protein to form puncta. We provide evidence that Nopp140 has condensation capacity and is required for CB assembly. From these observations, we propose a model in which coilin NTD-NTD mediated assemblies make multivalent contacts with Nopp140 to achieve biomolecular condensation in the nucleus.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Residual Cajal bodies in coilin knockout mice fail to recruit Sm snRNPs and SMN, the spinal muscular atrophy gene product.J Cell Biol. 2001 Jul 23;154(2):293-307. doi: 10.1083/jcb.200104083. J Cell Biol. 2001. PMID: 11470819 Free PMC article.
-
Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies.Mol Biol Cell. 2006 Jul;17(7):3221-31. doi: 10.1091/mbc.e06-03-0247. Epub 2006 May 10. Mol Biol Cell. 2006. PMID: 16687569 Free PMC article.
-
Control of Cajal body number is mediated by the coilin C-terminus.J Cell Sci. 2003 Jan 15;116(Pt 2):303-12. doi: 10.1242/jcs.00211. J Cell Sci. 2003. PMID: 12482916
-
Cajal bodies in neurons.RNA Biol. 2017 Jun 3;14(6):712-725. doi: 10.1080/15476286.2016.1231360. Epub 2016 Sep 14. RNA Biol. 2017. PMID: 27627892 Free PMC article. Review.
-
Coilin: The first 25 years.RNA Biol. 2015;12(6):590-6. doi: 10.1080/15476286.2015.1034923. RNA Biol. 2015. PMID: 25970135 Free PMC article. Review.
Cited by
-
How intrinsically disordered proteins order plant gene silencing.Trends Genet. 2024 Mar;40(3):260-275. doi: 10.1016/j.tig.2023.12.009. Epub 2024 Jan 30. Trends Genet. 2024. PMID: 38296708 Review.
-
Biomolecular condensates and disease pathogenesis.Sci China Life Sci. 2024 Sep;67(9):1792-1832. doi: 10.1007/s11427-024-2661-3. Epub 2024 Jul 17. Sci China Life Sci. 2024. PMID: 39037698 Review.
-
Coilin and Cajal bodies.Nucleus. 2023 Dec;14(1):2256036. doi: 10.1080/19491034.2023.2256036. Nucleus. 2023. PMID: 37682044 Free PMC article. Review.
-
Biomolecular condensates in plant RNA silencing: insights into formation, function, and stress responses.Plant Cell. 2024 Jan 30;36(2):227-245. doi: 10.1093/plcell/koad254. Plant Cell. 2024. PMID: 37772963 Free PMC article. Review.
-
Formation, function, and pathology of RNP granules.Cell. 2023 Oct 26;186(22):4737-4756. doi: 10.1016/j.cell.2023.09.006. Cell. 2023. PMID: 37890457 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

