Design, Formulation, and Evaluation of Aloe vera Gel-Based Capsaicin Transemulgel for Osteoarthritis
- PMID: 36145560
- PMCID: PMC9503439
- DOI: 10.3390/pharmaceutics14091812
Design, Formulation, and Evaluation of Aloe vera Gel-Based Capsaicin Transemulgel for Osteoarthritis
Abstract
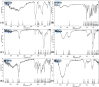
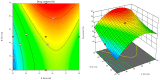
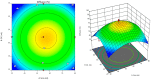
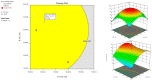
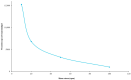
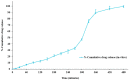
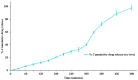
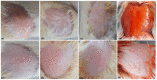
Topical treatments are a potential therapeutic option for the therapy of osteoarthritis, with significant data supporting the effectiveness and safety of topical formulation. Topical gel formulations may offer an alternative to oral formulations to relieve osteoarthritis (OA) pain while decreasing systemic exposure. Topical capsaicin transemulgel may represent an effective and safe alternative. The transemulgel was prepared from aqueous Aloe vera gel and Carbopol 934 with capsaicin in clove oil emulsion. The optimized transemulgel of capsaicin showed a pH of 6.1 ± 0.1 and viscosity of 15263-998 cps. Data from in vitro diffusion demonstrated improved permeability properties. The formulation caused no skin irritation when applied topically. The optimal transemulgel spreadability was found to be 20.23 g·cm/s. In vitro and ex vivo studies of the optimized formulation were performed. The skin irritant test was performed on rat skin with an optimized and marketed formulation. Both showed no irritation on the skin. The transemulgel of the capsaicin with Aloe vera gel was proven to be effective for osteoarthritis therapy.
Keywords: anti-inflammatory agent; arthritis; capsaicin; drug delivery; emulsion; topical.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Formulation and Characterization of Aceclofenac -Aloe vera Transemulgel.Curr Drug Deliv. 2015;12(6):703-8. doi: 10.2174/156720181206151228113209. Curr Drug Deliv. 2015. PMID: 25751659
-
In-vitro assessment and pharmacodynamics of nimesulide incorporated Aloe vera transemulgel.Curr Drug Discov Technol. 2014 Jun;11(2):162-7. doi: 10.2174/1570163810666131202233721. Curr Drug Discov Technol. 2014. PMID: 24295369
-
Liposomal Aloe vera trans-emulgel drug delivery of naproxen and nimesulide: A study.Int J Pharm Investig. 2015 Jan-Mar;5(1):28-34. doi: 10.4103/2230-973X.147230. Int J Pharm Investig. 2015. PMID: 25599030 Free PMC article.
-
Topical therapies for osteoarthritis.Drugs. 2011 Jul 9;71(10):1259-79. doi: 10.2165/11592550-000000000-00000. Drugs. 2011. PMID: 21770475 Review.
-
Topical therapies in the management of chronic pain.Postgrad Med. 2013 Jul;125(4 Suppl 1):25-33. doi: 10.1080/00325481.2013.1110567111. Postgrad Med. 2013. PMID: 24547601 Review.
Cited by
-
Preparation, Properties and Therapeutic Effect of a TPL Nanoparticle Thermosensitive Gel for Intra-Articular Injection.Molecules. 2023 Jun 9;28(12):4659. doi: 10.3390/molecules28124659. Molecules. 2023. PMID: 37375214 Free PMC article.
-
Aloe vera-Based Hydrogels for Wound Healing: Properties and Therapeutic Effects.Gels. 2023 Jul 3;9(7):539. doi: 10.3390/gels9070539. Gels. 2023. PMID: 37504418 Free PMC article. Review.
-
Overview of Anti-Inflammatory and Anti-Nociceptive Effects of Polyphenols to Halt Osteoarthritis: From Preclinical Studies to New Clinical Insights.Int J Mol Sci. 2022 Dec 13;23(24):15861. doi: 10.3390/ijms232415861. Int J Mol Sci. 2022. PMID: 36555503 Free PMC article. Review.
-
Formulation and Optimization of Solid Lipid Nanoparticle-based Gel for Dermal Delivery of Linezolid using Taguchi Design.Recent Adv Antiinfect Drug Discov. 2024;19(4):322-347. doi: 10.2174/0127724344280309240103062810. Recent Adv Antiinfect Drug Discov. 2024. PMID: 38243985
-
Development and evaluation of pH sensitive semi-interpenetrating networks: assessing the impact of itaconic acid and aloe vera on network swelling and cetirizine release.Front Bioeng Biotechnol. 2023 May 9;11:1173883. doi: 10.3389/fbioe.2023.1173883. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37229490 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

