Recent advances in therapeutic strategies for triple-negative breast cancer
- PMID: 36038913
- PMCID: PMC9422136
- DOI: 10.1186/s13045-022-01341-0
Recent advances in therapeutic strategies for triple-negative breast cancer
Abstract
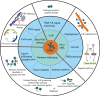
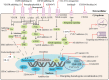
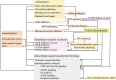
Triple-negative breast cancer (TNBC) is the most malignant subtype of breast cancer (BC) with a poor prognosis. Current treatment options are limited to surgery, adjuvant chemotherapy and radiotherapy; however, a proportion of patients have missed the surgical window at the time of diagnosis. TNBC is a highly heterogeneous cancer with specific mutations and aberrant activation of signaling pathways. Hence, targeted therapies, such as those targeting DNA repair pathways, androgen receptor signaling pathways, and kinases, represent promising treatment options against TNBC. In addition, immunotherapy has also been demonstrated to improve overall survival and response in TNBC. In this review, we summarize recent key advances in therapeutic strategies based on molecular subtypes in TNBC.
Keywords: Combination therapy; Immunotherapy; Targeted therapy; Triple-negative breast cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
An overview of triple-negative breast cancer.Arch Gynecol Obstet. 2016 Feb;293(2):247-69. doi: 10.1007/s00404-015-3859-y. Epub 2015 Sep 4. Arch Gynecol Obstet. 2016. PMID: 26341644 Review.
-
Immunotherapy for triple-negative breast cancer: Existing challenges and exciting prospects.Drug Resist Updat. 2017 May;32:1-15. doi: 10.1016/j.drup.2017.07.002. Epub 2017 Aug 19. Drug Resist Updat. 2017. PMID: 29145974 Review.
-
Targeting triple-negative breast cancer: A clinical perspective.Oncol Res. 2023 May 24;31(3):221-238. doi: 10.32604/or.2023.028525. eCollection 2023. Oncol Res. 2023. PMID: 37305385 Free PMC article. Review.
-
Immunotherapeutic interventions of Triple Negative Breast Cancer.J Transl Med. 2018 May 30;16(1):147. doi: 10.1186/s12967-018-1514-7. J Transl Med. 2018. PMID: 29848327 Free PMC article. Review.
-
Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer.J Immunother Cancer. 2021 Mar;9(3):e002022. doi: 10.1136/jitc-2020-002022. J Immunother Cancer. 2021. PMID: 33753567 Free PMC article.
Cited by
-
Deciphering breast cancer dynamics: insights from single-cell and spatial profiling in the multi-omics era.Biomark Res. 2024 Sep 18;12(1):107. doi: 10.1186/s40364-024-00654-1. Biomark Res. 2024. PMID: 39294728 Free PMC article. Review.
-
Recent Progress of Multifunctional Molecular Probes for Triple-Negative Breast Cancer Theranostics.Pharmaceutics. 2024 Jun 14;16(6):803. doi: 10.3390/pharmaceutics16060803. Pharmaceutics. 2024. PMID: 38931924 Free PMC article. Review.
-
Local, Sustained, and Targeted Co-Delivery of MEK Inhibitor and Doxorubicin Inhibits Tumor Progression in E-Cadherin-Positive Breast Cancer.Pharmaceutics. 2024 Jul 25;16(8):981. doi: 10.3390/pharmaceutics16080981. Pharmaceutics. 2024. PMID: 39204325 Free PMC article.
-
Simultaneous targeting and suppression of heat shock protein 60 to overcome heat resistance and induce mitochondrial death of tumor cells in photothermal immunotherapy.Mater Today Bio. 2024 Sep 29;29:101282. doi: 10.1016/j.mtbio.2024.101282. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39415762 Free PMC article.
-
Proteomic Markers for Mechanobiological Properties of Metastatic Cancer Cells.Int J Mol Sci. 2023 Mar 1;24(5):4773. doi: 10.3390/ijms24054773. Int J Mol Sci. 2023. PMID: 36902201 Free PMC article. Review.
References
-
- Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176–198. doi: 10.1158/2159-8290.CD-18-1177. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

