Transient receptor potential melastatin 3 dysfunction in post COVID-19 condition and myalgic encephalomyelitis/chronic fatigue syndrome patients
- PMID: 35986236
- PMCID: PMC9388968
- DOI: 10.1186/s10020-022-00528-y
Transient receptor potential melastatin 3 dysfunction in post COVID-19 condition and myalgic encephalomyelitis/chronic fatigue syndrome patients
Abstract
Background: Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a severe multisystemic condition associated with post-infectious onset, impaired natural killer (NK) cell cytotoxicity and impaired ion channel function, namely Transient Receptor Potential Melastatin 3 (TRPM3). Long-term effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has resulted in neurocognitive, immunological, gastrointestinal, and cardiovascular manifestations recently recognised as post coronavirus disease 2019 (COVID-19) condition. The symptomatology of ME/CFS overlaps significantly with post COVID-19; therefore, this research aimed to investigate TRPM3 ion channel function in post COVID-19 condition patients.
Methods: Whole-cell patch-clamp technique was used to measure TRPM3 ion channel activity in isolated NK cells of N = 5 ME/CFS patients, N = 5 post COVID-19 patients, and N = 5 healthy controls (HC). The TRPM3 agonist, pregnenolone sulfate (PregS) was used to activate TRPM3 function, while ononetin was used as a TRPM3 antagonist.
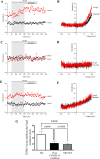
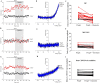
Results: As reported in previous research, PregS-induced TRPM3 currents were significantly reduced in ME/CFS patients compared with HC (p = 0.0048). PregS-induced TRPM3 amplitude was significantly reduced in post COVID-19 condition compared with HC (p = 0.0039). Importantly, no significant difference was reported in ME/CFS patients compared with post COVID-19 condition as PregS-induced TRPM3 currents of post COVID-19 condition patients were similar of ME/CFS patients currents (p > 0.9999). Isolated NK cells from post COVID-19 condition and ME/CFS patients were resistant to ononetin and differed significantly with HC (p < 0.0001).
Conclusion: The results of this investigation suggest that post COVID-19 condition patients may have impaired TRPM3 ion channel function and provide further evidence regarding the similarities between post COVID-19 condition and ME/CFS. Impaired TRPM3 channel activity in post COVID-19 condition patients suggest impaired ion mobilisation which may consequently impede cell function resulting in chronic post-infectious symptoms. Further investigation into TRPM3 function may elucidate the pathomechanism, provide a diagnostic and therapeutic target for post COVID-19 condition patients and commonalities with ME/CFS patients.
Keywords: Coronavirus; Myalgic encephalomyelitis/chronic fatigue syndrome; Natural killer cells; Post COVID-19 condition; SARS-CoV-2; Transient receptor potential melastatin 3; Whole-cell patch clamp electrophysiology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Investigation into the restoration of TRPM3 ion channel activity in post-COVID-19 condition: a potential pharmacotherapeutic target.Front Immunol. 2024 May 3;15:1264702. doi: 10.3389/fimmu.2024.1264702. eCollection 2024. Front Immunol. 2024. PMID: 38765011 Free PMC article.
-
Validation of impaired Transient Receptor Potential Melastatin 3 ion channel activity in natural killer cells from Chronic Fatigue Syndrome/ Myalgic Encephalomyelitis patients.Mol Med. 2019 Apr 23;25(1):14. doi: 10.1186/s10020-019-0083-4. Mol Med. 2019. PMID: 31014226 Free PMC article.
-
The effect of IL-2 stimulation and treatment of TRPM3 on channel co-localisation with PIP2 and NK cell function in myalgic encephalomyelitis/chronic fatigue syndrome patients.J Transl Med. 2021 Jul 15;19(1):306. doi: 10.1186/s12967-021-02974-4. J Transl Med. 2021. PMID: 34266470 Free PMC article.
-
Potential pathophysiological role of the ion channel TRPM3 in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and the therapeutic effect of low-dose naltrexone.J Transl Med. 2024 Jul 5;22(1):630. doi: 10.1186/s12967-024-05412-3. J Transl Med. 2024. PMID: 38970055 Free PMC article. Review.
-
Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome.J Adv Res. 2022 Sep;40:179-196. doi: 10.1016/j.jare.2021.11.013. Epub 2021 Nov 26. J Adv Res. 2022. PMID: 36100326 Free PMC article. Review.
Cited by
-
ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature.Front Med (Lausanne). 2023 Jun 2;10:1187163. doi: 10.3389/fmed.2023.1187163. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37342500 Free PMC article. Review.
-
A Proposed New Model to Explain the Role of Low Dose Non-DNA Targeted Radiation Exposure in Chronic Fatigue and Immune Dysfunction Syndrome.Int J Mol Sci. 2023 Mar 23;24(7):6022. doi: 10.3390/ijms24076022. Int J Mol Sci. 2023. PMID: 37046994 Free PMC article. Review.
-
Hypothesis: inflammatory acid-base disruption underpins Long Covid.Front Immunol. 2023 Apr 14;14:1150105. doi: 10.3389/fimmu.2023.1150105. eCollection 2023. Front Immunol. 2023. PMID: 37122723 Free PMC article.
-
Brainstem volume changes in myalgic encephalomyelitis/chronic fatigue syndrome and long COVID patients.Front Neurosci. 2023 Mar 2;17:1125208. doi: 10.3389/fnins.2023.1125208. eCollection 2023. Front Neurosci. 2023. PMID: 36937672 Free PMC article.
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
References
-
- Anasetti C, Martin PJ, June CH, Hellstrom KE, Ledbetter JA, Rabinovitch PS, et al. Induction of calcium flux and enhancement of cytolytic activity in natural killer cells by cross-linking of the sheep erythrocyte binding protein (CD2) and the Fc-receptor (CD16) J Immunol. 1987;139(6):1772–1779. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

