Impact of the COVID-19 pandemic on hepatitis B and C elimination: An EASL survey
- PMID: 35967191
- PMCID: PMC9364666
- DOI: 10.1016/j.jhepr.2022.100531
Impact of the COVID-19 pandemic on hepatitis B and C elimination: An EASL survey
Abstract
Background & aims: The World Health Organization (WHO) HBV and HCV elimination targets, set in 2016 and based on projections to 2030, were unable to consider the impact of intervening factors. To evaluate the impact of the COVID-19 pandemic on viral hepatitis elimination programs, the European Association for the Study of the Liver (EASL) conducted a survey in liver centers worldwide in 2021.
Methods: A web-based questionnaire was distributed (May-July 2021) to all EASL members representing clinical units providing HBV and HCV hepatitis care. Results are expressed as absolute numbers and reduction rates for each care activity.
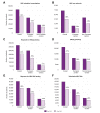
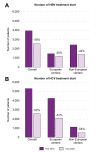
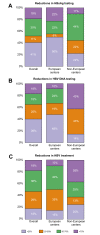
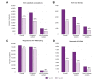
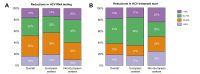
Results: Data were collected from 32 European and 12 non-European clinical centers. Between January 2019 (pre-pandemic) and December 2020 (during the pandemic), chronic HBV consultations decreased by 32% and 26%, new referrals by 38% and 39%, HBV testing rates by 39% and 21% (for HBsAg detection) and 30% and 22% (for HBV DNA detection), and new HBV treatments by 20% and 44% (p = 0.328) in European and non-European centers, respectively. With regard to HCV during the same time frame, the overall reductions were 39% and 50% for consultations, 49% and 49% for new referrals, 11% and 38% for HCV RNA detection, and 51% and 54% for new HCV antiviral treatments for European and non-European Centers, respectively (p = 0.071).
Conclusions: All steps in the viral hepatitis care cascade have been hampered by the COVID-19 pandemic, with a comparable impact across different centers. These data reaffirm the pandemic's major effect on global viral hepatitis elimination programs and suggest that actions to achieve the WHO 2030 targets should be reconsidered and revised to account for each country's progress relative to pre-pandemic values.
Lay summary: The EASL multinational survey conclusively shows that viral hepatitis elimination programs, expected to provide control of hepatitis B and hepatitis C worldwide by 2030, have been held back by the COVID-19 pandemic in clinical centers from several European and non-European countries, with a comparable impact across centers. Limitations in the cascade of care for both HBV and HCV were linked to limited access to screening, consultations, specific testing, and actual treatment. As restrictions for COVID-19 begin to lift, efforts to diagnose and provide treatment for viral hepatitis should remain high on the list of priorities for public health officials to maintain the WHO elimination efforts. Measures that have been put in place to control the COVID-19 pandemic could be transferred to increasing the diagnosis and linkage to care of people with hepatitis.
Keywords: COVID-19 pandemic; EASL, European Association for the Study of the Liver; EU Centers, European centers; Hepatitis B virus; Hepatitis C virus; Non-EU Centers, Non-European centers; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; WHO elimination targets; WHO, World Health Organization.
© 2022 Published by Elsevier B.V. on behalf of European Association for the Study of the Liver (EASL).
Conflict of interest statement
LK: Received speaker fees from Gilead and Abbvie and consultancy fees from Abbvie. MB: Received grants/research support from Abbvie, BMS, and Gilead. Received honoraria/consultation fees or participated in an advisory board for Abbvie, Gilead, GSK, Janssen, MSD, and Roche. Participated in a company-sponsored speaker’s bureau for Abbvie, Gilead, and Janssen. MRB: Received research funding from Gilead and served as a speaker for Gilead and AbbVie. MM: Nothing to declare. TB: Received grants/research support from Abbvie, BMS, Gilead, MSD/Merck, Humedics, Intercept, Merz, Norgine, Novartis, Orphalan, and Sequana Medical. Received honoraria/consultation fees or served on the advisory board for Abbvie, Alexion, Bayer, Gilead, GSK, Eisai, Enyo Pharma, Falk Foundation, HepaRegeniX GmbH, Humedics, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Orphalan, Roche, Sequana Medical, SIRTEX, SOBI, and Shionogi. Participated in a company-sponsored speaker’s bureau for Abbvie, Alexion, Bayer, Gilead, Eisai, Intercept, Ipsen, Janssen, MedUpdate GmbH, MSD/Merck, Novartis, Orphalan, Sequana Medica, SIRTEX, and SOBI. FN: Advisor for Gilead and AbbVie, and received speaker fees from Roche Diagnostics. AC: Received grants and research support from AbbVie, Alfasigma, Bayer, BMS, Gilead Sciences, Intercept, MSD, and Roche. Served on advisory committees for AbbVie, Alfasigma, Bayer, BMS, Gilead Sciences, Intercept, MSD, and Roche. Participated in speaking and teaching activities for AbbVie, Alfasigma, Bayer, BMS, and Gilead Sc. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Similar articles
-
Impact of Coronavirus Disease 2019 Pandemic on Viral Hepatitis Elimination: What Is the Price?AIDS Res Hum Retroviruses. 2021 Aug;37(8):585-588. doi: 10.1089/AID.2020.0301. Epub 2021 Jun 2. AIDS Res Hum Retroviruses. 2021. PMID: 33913731 Free PMC article.
-
The impact of the first, second and third waves of covid-19 on hepatitis B and C testing in Ontario, Canada.J Viral Hepat. 2022 Mar;29(3):205-208. doi: 10.1111/jvh.13637. Epub 2021 Dec 5. J Viral Hepat. 2022. PMID: 34820967
-
Absolute targets for HCV elimination and national health policy paradigms: Foreseeing future requirements.Liver Int. 2021 Apr;41(4):649-655. doi: 10.1111/liv.14796. Epub 2021 Feb 8. Liver Int. 2021. PMID: 33486885 Review.
-
The case for simplifying and using absolute targets for viral hepatitis elimination goals.J Viral Hepat. 2021 Jan;28(1):12-19. doi: 10.1111/jvh.13412. Epub 2020 Nov 4. J Viral Hepat. 2021. PMID: 32979881
-
Hepatitis B Gene Therapy Coming to Age.AIDS Rev. 2018 Apr-Jun;20(2):125-127. AIDS Rev. 2018. PMID: 29938706 Review.
Cited by
-
World Hepatitis Day in 2022: Challenges of Viral Hepatitis Elimination in Elongated COVID-19 Pandemic.Pathogens. 2022 Sep 1;11(9):1002. doi: 10.3390/pathogens11091002. Pathogens. 2022. PMID: 36145433 Free PMC article.
-
Impact of COVID-19 on epidemic trend of hepatitis C in Henan Province assessed by interrupted time series analysis.BMC Infect Dis. 2023 Oct 17;23(1):691. doi: 10.1186/s12879-023-08635-9. BMC Infect Dis. 2023. PMID: 37848842 Free PMC article.
-
World Hepatitis Day 2023: Are we close to the target?Indian J Med Res. 2023 Jan;158(1):1-4. doi: 10.4103/ijmr.ijmr_1250_23. Indian J Med Res. 2023. PMID: 37602579 Free PMC article. No abstract available.
-
Global epidemiology of cirrhosis - aetiology, trends and predictions.Nat Rev Gastroenterol Hepatol. 2023 Jun;20(6):388-398. doi: 10.1038/s41575-023-00759-2. Epub 2023 Mar 28. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36977794 Free PMC article. Review.
-
Optimizing Hepatitis C Treatment Monitoring: Is Sustained Virologic Response at 4 Weeks Becoming the New Standard?Microorganisms. 2024 Oct 10;12(10):2050. doi: 10.3390/microorganisms12102050. Microorganisms. 2024. PMID: 39458359 Free PMC article.
References
-
- Aghemo A., Masarone M., Montagnese S., Petta S., Ponziani F.R., Russo F.P., et al. Assessing the impact of COVID-19 on the management of patients with liver diseases: a national survey by the Italian association for the study of the liver. Dig Liver Dis. 2020;52:937–941. doi: 10.1016/j.dld.2020.07.008. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

