Comparative Effectiveness of Opioid Tapering or Abrupt Discontinuation vs No Dosage Change for Opioid Overdose or Suicide for Patients Receiving Stable Long-term Opioid Therapy
- PMID: 35960518
- PMCID: PMC9375167
- DOI: 10.1001/jamanetworkopen.2022.26523
Comparative Effectiveness of Opioid Tapering or Abrupt Discontinuation vs No Dosage Change for Opioid Overdose or Suicide for Patients Receiving Stable Long-term Opioid Therapy
Abstract
Importance: Opioid dosage tapering has emerged as a strategy to reduce harms associated with long-term opioid therapy; however, evidence supporting this approach is limited.
Objective: To identify the association of opioid tapering or abrupt discontinuation with opioid overdose and suicide events among patients receiving stable long-term opioid therapy without evidence of opioid misuse.
Design, setting, and participants: This comparative effectiveness study with a trial emulation approach used a large US claims data set of individuals with commercial insurance or Medicare Advantage who were aged 18 years or older and receiving stable long-term opioid therapy without evidence of opioid misuse between January 1, 2010, and December 31, 2018. Statistical analysis was performed from January 17, 2020, through November 12, 2021.
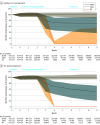
Interventions: Three opioid dosage strategies: stable dosage, tapering (dosage reduction ≥15%), or abrupt discontinuation.
Main outcomes and measures: Time to opioid overdose or suicide event identified from International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes in medical claims over 11 months of follow-up. Inverse probability weighting was used to adjust for baseline confounders. The primary analysis used an intention-to-treat approach; follow-up after assignment regardless of changes in opioid dose was included. A per-protocol analysis was also conducted, in which episodes were censored for lack of adherence to assigned treatment.
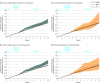
Results: A cohort of 199 836 individuals (45.1% men; mean [SD] age, 56.9 [12.4] years; and 57.6% aged 45-64 years) had 415 123 qualifying, long-term opioid therapy episodes; 87.1% of episodes were considered stable, 11.1% were considered a taper, and 1.8% were considered abrupt discontinuation. The adjusted cumulative incidence of opioid overdose or suicide events 11 months after baseline was 0.96% (95% CI, 0.92%-0.99%) with a stable dosage strategy, 1.10% (95% CI, 0.99%-1.22%) with a tapered dosage strategy, and 1.28% (95% CI, 0.93%-1.38%) with an abrupt discontinuation strategy. The risk difference between a taper and a stable dosage was 0.15% (95% CI, 0.03%-0.26%), and the risk difference between abrupt discontinuation and a stable dosage was 0.33% (95% CI, -0.03% to 0.74%). Results were similar using the per-protocol approach.
Conclusions and relevance: This study identified a small absolute increase in risk of harms associated with opioid tapering compared with a stable opioid dosage. These results do not suggest that policies of mandatory dosage tapering for individuals receiving a stable long-term opioid dosage without evidence of opioid misuse will reduce short-term harm via suicide and overdose.
Conflict of interest statement
Figures

Similar articles
-
Long-term Risk of Overdose or Mental Health Crisis After Opioid Dose Tapering.JAMA Netw Open. 2022 Jun 1;5(6):e2216726. doi: 10.1001/jamanetworkopen.2022.16726. JAMA Netw Open. 2022. PMID: 35696163 Free PMC article.
-
Association of Opioid Dose Reduction With Opioid Overdose and Opioid Use Disorder Among Patients Receiving High-Dose, Long-term Opioid Therapy in North Carolina.JAMA Netw Open. 2022 Apr 1;5(4):e229191. doi: 10.1001/jamanetworkopen.2022.9191. JAMA Netw Open. 2022. PMID: 35476064 Free PMC article.
-
Benzodiazepine Discontinuation and Mortality Among Patients Receiving Long-Term Benzodiazepine Therapy.JAMA Netw Open. 2023 Dec 1;6(12):e2348557. doi: 10.1001/jamanetworkopen.2023.48557. JAMA Netw Open. 2023. PMID: 38117495 Free PMC article.
-
Opioid Treatments for Chronic Pain [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Apr. Report No.: 20-EHC011. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Apr. Report No.: 20-EHC011. PMID: 32338848 Free Books & Documents. Review.
-
Assessing Variation in State Opioid Tapering Laws: Comparing State Laws with the CDC Guideline.Pain Med. 2021 Dec 11;22(12):2941-2949. doi: 10.1093/pm/pnab208. Pain Med. 2021. PMID: 34196723 Review.
Cited by
-
Predictors of fatal and nonfatal overdose after prescription of opioids for chronic pain: a systematic review and meta-analysis of observational studies.CMAJ. 2023 Oct 23;195(41):E1399-E1411. doi: 10.1503/cmaj.230459. CMAJ. 2023. PMID: 37871953 Free PMC article.
-
Update of a Multivariable Opioid Overdose Risk Prediction Model to Enhance Clinical Care for Long-term Opioid Therapy Patients.J Gen Intern Med. 2023 Sep;38(12):2678-2685. doi: 10.1007/s11606-023-08149-9. Epub 2023 Mar 21. J Gen Intern Med. 2023. PMID: 36944901 Free PMC article.
-
Experiences and Outcomes of Using e-Prescribing for Opioids: Rapid Scoping Review.J Med Internet Res. 2023 Dec 28;25:e49173. doi: 10.2196/49173. J Med Internet Res. 2023. PMID: 38153776 Free PMC article.
-
Reducing Opioid Use for Chronic Pain With a Group-Based Intervention: A Randomized Clinical Trial.JAMA. 2023 May 23;329(20):1745-1756. doi: 10.1001/jama.2023.6454. JAMA. 2023. PMID: 37219554 Free PMC article. Clinical Trial.
-
Opioid Monitoring in Clinical Settings: Strategies and Implications of Tailored Approaches for Therapy.Int J Mol Sci. 2024 May 29;25(11):5925. doi: 10.3390/ijms25115925. Int J Mol Sci. 2024. PMID: 38892112 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

