Transient Receptor Potential Vanilloid-1 (TRPV1) Alleviates Hepatic Fibrosis via TGF- β Signaling
- PMID: 35909891
- PMCID: PMC9334033
- DOI: 10.1155/2022/3100943
Transient Receptor Potential Vanilloid-1 (TRPV1) Alleviates Hepatic Fibrosis via TGF- β Signaling
Abstract
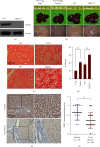
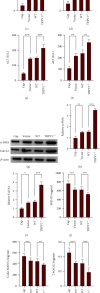
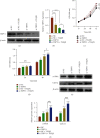
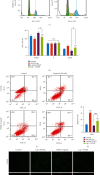
Hepatic fibrosis is a major global health problem and considered a leading cause of liver-related morbidity and mortality worldwide. Although previous studies have suggested that transient receptor potential vanilloid-1 (TRPV1) is protective against cardiac and renal fibrosis, its functional role in hepatic fibrosis has remained elusive. Herein, we characterize the effects of TRPV1 on carbon tetrachloride- (CCl4-) induced mice, in vitro transforming growth factor-β- (TGF-β-) treated hepatic stellate cells (HSCs), and human fibrosis specimens. Finally, our results demonstrated the significant TRPV1 downregulation in human liver fibrosis tissues. Knocking out TRPV1 significantly increased the expression of various hepatic fibrosis markers, while the expression of these biomarkers declined markedly in capsaicin-activated mice. Moreover, our study revealed that knocking down TRPV1 would enhance the promotive effect of TGF-β on HSC proliferation, cell cycle, cell apoptosis, and ECM expression. Also, such promotive effect can be partially reversible by capsaicin, an exogenous activator of TRPV1. Collectively, the obtained data suggest that TRPV1 may alleviate CCl4-induced hepatic fibrosis and attenuate the effect of TGF-β on HSC activation, proliferation, and apoptosis, which overall implies that targeting TRPV1 channel activity may be an effective therapeutic strategy for treating hepatic fibrosis.
Copyright © 2022 Ke Qian et al.
Conflict of interest statement
The authors declared no conflict of interest.
Figures
Similar articles
-
CAT1 silencing inhibits TGF-β1-induced mouse hepatic stellate cell activation in vitro and hepatic fibrosis in vivo.Cytokine. 2020 Dec;136:155288. doi: 10.1016/j.cyto.2020.155288. Epub 2020 Sep 25. Cytokine. 2020. PMID: 32980687
-
Thymosin β4 suppresses CCl4 -induced murine hepatic fibrosis by down-regulating transforming growth factor β receptor-II.J Gene Med. 2018 Sep;20(9):e3043. doi: 10.1002/jgm.3043. Epub 2018 Aug 15. J Gene Med. 2018. PMID: 29972714
-
Capsaicin receptor TRPV1 maintains quiescence of hepatic stellate cells in the liver via recruitment of SARM1.J Hepatol. 2023 Apr;78(4):805-819. doi: 10.1016/j.jhep.2022.12.031. Epub 2023 Jan 18. J Hepatol. 2023. PMID: 36669703
-
Gli2-regulated activation of hepatic stellate cells and liver fibrosis by TGF-β signaling.Am J Physiol Gastrointest Liver Physiol. 2021 May 1;320(5):G720-G728. doi: 10.1152/ajpgi.00310.2020. Epub 2021 Mar 17. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33728992
-
Transient receptor potential vanilloid subtype 1: A potential therapeutic target for fibrotic diseases.Front Physiol. 2022 Aug 15;13:951980. doi: 10.3389/fphys.2022.951980. eCollection 2022. Front Physiol. 2022. PMID: 36045746 Free PMC article. Review.
Cited by
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
-
The Role of Cannabidiol in Liver Disease: A Systemic Review.Int J Mol Sci. 2024 Feb 17;25(4):2370. doi: 10.3390/ijms25042370. Int J Mol Sci. 2024. PMID: 38397045 Free PMC article. Review.
-
Interference of periostin attenuates pathological changes, proinflammatory markers and renal fibrosis in diabetic kidney injury.Genes Genomics. 2023 Nov;45(11):1389-1397. doi: 10.1007/s13258-023-01400-x. Epub 2023 May 29. Genes Genomics. 2023. PMID: 37248423
-
Role of TRP Channels in Metabolism-Related Diseases.Int J Mol Sci. 2024 Jan 5;25(2):692. doi: 10.3390/ijms25020692. Int J Mol Sci. 2024. PMID: 38255767 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

